Abstract
The poisoning of the environment is currently one of the most important concerns on a global scale. Polycyclic aromatic hydrocarbons, also known as PAHs, are one of the most difficult problems associated with the indemnification of the hydrosphere. This issue was caused by the inadvertent release of reactive chemicals into the environment. Prepared nanocellulose was modified with titanium oxide. Using TiO2@nanocellulose as chemical adsorbents, PAHs, like naphthalene and pyrene, were effectively removed from aqueous solution. The structural and morphological properties of the synthesized adsorbents were determined through XRD, FTIR, FE-SEM, and BET analysis. From the results, it was confirmed that titanium was successfully loaded onto the surface of nanocellulose. At low pH, naphthalene and pyrene were eliminated most effectively after 120 min. Thermodynamic, error analysis, kinetic, and isotherm models were applied to the experimental data. The error analysis showed that the sorption process was well described by the pseudo-second-order and Langmuir models. Nearly 84.3% and 73% of the naphthalene and pyrene, respectively, were eliminated at its peak removal efficiency. The effectiveness of TiO2@nanocellulose in terms of desorption and reuse was also studied. This research suggests that the presence of active sites and increased surface area of TiO2@nanocellulose increases its removal capacity for naphthalene and pyrene.
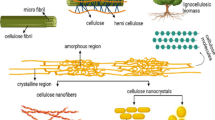
Graphical abstract





Similar content being viewed by others
Data availability
Will be made available upon reasonable request.
References
Adeola AO, Forbes PBC (2020) Advances in water treatment technologies for removal of polycyclic aromatic hydrocarbons: existing concepts, emerging trends, and future prospects. Water Environ Res 93(3):343–359. https://doi.org/10.1002/wer.1420
Ahmed MA, Mohamed AA (2023) A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants. Inorg Chem Commun 148:110325
Ambade B, Kumar A, Kumar A, Sahu LK (2022) Temporal variability of atmospheric particulate-bound polycyclic aromatic hydrocarbons (PAHs) over central east India: sources and carcinogenic risk assessment. Air Qual Atmos Health 15:115–130
Aravind M, Amalanathan M, Mary MSM (2021) Synthesis of TiO 2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl Sci 3:1–10
Arularasu MV, Harb M, Sundaram R (2020) Synthesis and characterization of cellulose/TiO2 nanocomposite: evaluation of in vitro antibacterial and in silico molecular docking studies. Carbohyd Polym 249:116868
Chen J, Qin Y, Chen Z, Yang Z, Yang W, Wang Y (2016) Gas circulating fluidized beds photocatalytic regeneration of I-TiO2 modified activated carbons saturated with toluene. Chem Eng J 293:281–290
Chougala LS, Yatnatti MS, Linganagoudar RK, Kamble RR, Kadadevarmath JS (2017) A simple approach on synthesis of TiO2 nanoparticles and its application in dye sensitized solar cells.
Dutta V, Devasia J, Chauhan A, Jayalakshmi M, Vasantha VL, Jha A, Nizam A, Lin KYA, Ghotekar S (2022) Photocatalytic nanomaterials: applications for remediation of toxic polycyclic aromatic hydrocarbons and green management. Chem Eng J Adv 11:100353. https://doi.org/10.1016/j.ceja.2022.100353
Faboya OL, So**u SO, Oguntuase BJ, Sonibare OO (2020) Impact of forest fires on polycyclic aromatic hydrocarbon concentrations and stable carbon isotope compositions in burnt soils from tropical forest, Nigeria. Sci Afr 8:e00331
Farhadi S, Dakheli MJ (2023) Adsorption of polycyclic aromatic hydrocarbons (PAHs) by nano-material-reinforced fibrous casings in smoked sausages. Polymer Bull 1–16
Freundlich HM (1906) Over the adsorption in solution. J Phys Chem A 57:385–470
Gupta H (2015) Removal of phenanthrene from water using activated carbon developed from orange rind. Int J Sci Res Environ Sci 3(7):248
Gupta H, Gupta B (2016) Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin Water Treat 57(20):9498–9509
Gupta H, Kumar R (2016) Removal of PAH anthracene from aqueous media using banana peel activated carbon. Int J Sci Res Environ Sci 4(4):109–114
Gutierrez-Urbano I, Villen-Guzman M, Perez-Recuerda R, Rodriguez-Maroto JM (2021) Removal of polycyclic aromatic hydrocarbons (PAHs) in conventional drinking. Water Treat Process 243:103888. https://doi.org/10.1016/j.jconhyd.2021.103888
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hospodarova V, Singovszka E, Stevulova N (2018) Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am J Anal Chem 9(6):303–310
Kadri T, Rouissi T, Brar SK, Cledon M, Sarma S, Verma M (2017) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: a review. J Environ Sci 51:52–74. https://doi.org/10.1016/j.jes.2016.08.023
Kataria N, Chauhan AK, Garg VK, Kumar P (2022) Sequestration of heavy metals from contaminated water using magnetic carbon nanocomposites. J Hazardous Mater Adv 6:100066
Kaur M, Pal J (2023) Evaluation of efficiency of Wheat straw nanocellulose as nanoadsorbent for the removal of divalent copper, lead and zinc from aqueous solution. Carbohydr Polym Technol Appl 6:100350. https://doi.org/10.1016/j.carpta.2023.100350
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. KungligaSvenskaVetenskapsakademiensHandlingar 24:1–39.
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1368
Li S, Luo J, Hang X, Wan Y (2019) Removal of polycyclic aromatic hydrocarbons by nanofiltration membranes: Rejection and fouling mechanisms. J Membr Sci 582:264–273. https://doi.org/10.1016/j.memsci.2019.04.008
Li Y, Yan Z, Fan J, Yao X, Cai Y (2023) Preparation of COF-coated nickel foam adsorbents for dispersive solid-phase extraction of 16 polycyclic aromatic hydrocarbons from Chinese herbal medicines. Talanta 124916
Long C, Lu J, Li A, Hu D, Liu F, Zhang Q (2008) Adsorption of naphthalene onto the carbon adsorbent from waste ion exchange resin: equilibrium and kinetic characteristics. J Hazard Mater 150(3):656–661
Mallah MA, Changxing L, Mallah MA, Noreen S, Liu Y, Saeed M, Zhang Q (2022) Polycyclic aromatic hydrocarbon and its effects on human health: an updated review. Chemosphere 133948.
Mensah P, Osobamiro T, Ramasami P (2022) Simultaneous remediation of polycyclic aromatic hydrocarbon and heavy metals in wastewater with zerovalent iron-titanium oxide nanoparticles (ZVI-TiO2). Phys Sci Rev 8(13):5185–5185. https://doi.org/10.1515/psr-2021-0213
Onwuka KE, Atasie OC, Igwe JC, Ogbuehi GI, Okereke SOE, Eze KS (2021) Ezeaku II kinetic modelingsnd thermodynamic study of the simultaneous adsorption of polycyclic aromatic hydrocarbons (PAHs) onto organomontmorillonite in aqueous medium. Int J Compos Mater Matr 8(1):28–38p
Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D (2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol 11:562813
Poudel BR, Aryal RL, Bhattarai S, Koirala AR, Gautam SK, Ghimire KN, Pant B, Park M, Paudyal H, Pokhrel MR (2020) Agro-waste derived biomass impregnated with TiO2 as a potential adsorbent for removal of as (III) from water. Catal 10(10):1125
Queiroz RN, Prediger P, Vieira MGA (2022) Adsorption of polycyclic aromatic hydrocarbons from wastewater using graphene-based nanomaterials synthesized by conventional chemistry and green synthesis: a critical review. J Hazard Mater 422:126904
Rajakumar G, Rahuman AA, Roopan SM, Khanna VG, Elango G, Kamaraj C, Velayutham K (2012) Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A Mol Biomol Spectrosc 91:23–29
Rani M, Shanker U (2019) Degradation of tricyclic polyaromatic hydrocarbons in water, soil and river sediment with a novel TiO2 based heterogeneous nanocomposite. J Environ Manag 248:109340
Ranwala P, Pal J, Garg VK, Rani S (2023) Synthesis of nanocellulose for the removal of naphthalene from simulated wastewater. Chem Papers 1–11
Rasheed A, Farooq F, Rafique U, Nasreen S, Aqeel Ashraf M (2016) Analysis of sorption efficiency of activated carbon for removal of anthracene and pyrene for wastewater treatment. Desalin Water Treat 57(1):145–150
Rashid J, Barakat MA, Ruzmanova Y, Chianese A (2015) Fe3O4/SiO2/TiO2 nanoparticles for photocatalytic degradation of 2-chlorophenol in simulated wastewater. Environ Sci Pollut Res 22:3149–3157. https://doi.org/10.1007/s11356-014-3598-9
Rashid R, Shafiq I, Iqbal MJ, Shabir M, Akhter PM, Hamayun MH, Ahmed A, Hussain M (2021) Synergistic effect of NS co-doped TiO2 adsorbent for removal of cationic dyes. J Environ Chem Eng 9(4):105480. https://doi.org/10.1016/j.jece.2021.105480
Rayaroth MP, Marchel M, Boczkaj G (2023) Advanced oxidation processes for the removal of mono and polycyclic aromatic hydrocarbons-a review. Sci Total Environ 857(2):159043. https://doi.org/10.1016/j.scitotenv.2022.159043
Saini J, Garg VK, Gupta RK, Kataria N (2017) Removal of Orange G and Rhodamine B dyes from aqueous system using hydrothermally synthesized zinc oxide loaded activated carbon (ZnO-AC). J Environ Chem Eng 5(1):884–892
Shahi N, Wang P, Adhikari S, Min B, Rangari VK (2021) Biopolymers fractionation and synthesis of nanocellulose/silica nanoparticles from agricultural byproducts. ACS Sustain Chem Eng 9(18):6284–6295
Temkin M, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim URSS 12:217–222
Theivasanthi T, Alagar M (2013) Titanium dioxide (TiO2) nanoparticles XRD analyses: an insight. ar**v preprint ar**v:1307.1091
Tran CV, Nguyen PTH, Nguyen DD, Pham HT, Do DT, La DD (2023) Facile Fabrication of Fe2O3/TiO2 Composite from Titanium Slag as Adsorbent for As (V) Removal from Aqueous Media. Sustain 15(9):7253
Wang J, Cao W, Wei W, ** H (2023) Adsorption characteristic analysis of PAHs on activated carbon with different functional groups by molecular simulation. Environ Sci Pollut Res 30(12):32452–32463
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J Sanit Eng Div Am Soc Civil Eng 89:31–60
Zhang LY, Yang JJ, Han YL (2022) Novel adsorption-photocatalysis integrated bismuth tungstate modified layered mesoporous titanium dioxide (Bi2WO6/LM-TiO2) composites. Opt Mater 130:112581
Acknowledgements
The authors acknowledge the Central Instrumentation Laboratory, Central University of Punjab, Bhatinda, Punjab, for providing instrument facilities.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Jitender Pal and Pooja Ranwala conceptualized the presented idea. Pooja Ranwala performed all characterizations and experiments of adsorption studies. Pooja Ranwala and Jitender Pal have written the original manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The authors certify that this article does not contain any studies with human participants or animals performed by any of them.
Consent to participate
We consented.
Consent to publish
We consented.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11696_2024_3378_MOESM1_ESM.docx
Supplementary file 1: Fig. S1 a.) First order, b) Second order, and c) Intraparticle diffusion model of kinetic study of pyrene and napthalene removal by modified nanocellulose. Fig. S2 a) Langmiur, b) Freundlich, and c) Temkin models of isotherm at pyrene and napthalene removal by modified nanocellulose. (DOCX 200 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ranwala, P., Pal, J. Comparative investigation of naphthalene and pyrene adsorption by modified nanocellulose with titanium oxide. Chem. Pap. 78, 4175–4187 (2024). https://doi.org/10.1007/s11696-024-03378-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03378-6