Abstract
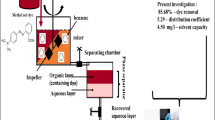
Liquid–liquid extraction has gained huge attention in chemical industries for the removal of valuable products. The aim of this research to optimize the percentage dye removal, distribution coefficient and solvent capacity for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene as an extractant. In this work, effect of parameters such as dye concentration in feed (20–100 ppm), extraction time (10–30 min), and dye solution to solvent ratio (1–3 mL/mL) was examined at constant pH, and temperature of 3, and 27 ± 2 °C. Response surface methodology (RSM)-based Box–Behnken design was used for optimization by considering independent and dependent factors. The optimization results reveal that the percentage dye removal, distribution coefficient and solvent capacity of 81.28%, 4.33 and 21.7 mg/L were achieved at dye concentration in feed, extraction time, and dye solution to solvent ratio of 45 ppm, 27 min, and 1.5 mL/mL, respectively. Thus, benzene could be the potential solvent for liquid–liquid extraction of methyl red dye from its aqueous solution.



Similar content being viewed by others
Abbreviations
- β o :
-
Intercept
- β i :
-
Linear coefficient
- β ii :
-
Squared coefficient
- β ij :
-
Interaction coefficient
- Y :
-
Response matrix
- X :
-
Input matrix
- ε :
-
Random error
- A :
-
Concentration of dye in feed
- B :
-
Extraction time
- C :
-
Dye solution to solvent ratio
- Y 1 :
-
Dye removal
- Y 2 :
-
Distribution coefficient
- Y 3 :
-
Solvent capacity
References
Abou-Hadid AF (2003) The use of saline water in agriculture in the Near East and North Africa Region: present and future. J Crop Prod 7(1–2):299–323
Asthana M, Kumar A, Sharma BS (2017) Wastewater treatment. In: Principles and applications of environmental biotechnology for a sustainable future. Springer, Singapore, pp 173–232
Bidari E, Irannejad M, Gharabaghi M (2013) Solvent extraction recovery and separation of cadmium and copper from sulphate solution. J Environ Chem Eng 1(4):1269–1274
Chandrasekaran AP, Sivamani S (2018) Statistical modeling and optimization of pretreatment for fermentable sugars production from cotton gin waste. Energy Sources Part a: Recov Util Environ Effects 40(4):400–405
Chang SH (2020) Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—a review. Environ Sci Pollut Res 27(26):32371–32388
De Castro ML, Garcıa-Ayuso LE (1998) Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta 369(1–2):1–10
Degryse F, Smolders E, Parker DR (2009) Partitioning of metals (Cd Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications—a review. Eur J Soil Sci 60(4):590–612
Fredj SB, Nobbs J, Tizaoui C, Monser L (2015) Removal of estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2) from wastewater by liquid–liquid extraction. Chem Eng J 262:417–426
Frick D (2003) The coloration of food. Rev Prog Color Relat Top 33(1):15–32
Huddleston JG, Willauer HD, Griffin ST, Rogers RD (1999) Aqueous polymeric solutions as environmentally benign liquid/liquid extraction media. Ind Eng Chem Res 38(7):2523–2539
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26(4):201–221
Joaquin AA, Nirmala G, Kanakasabai P (2021) Response surface analysis for sewage wastewater treatment using natural coagulants. Polish J Environ Stud 30(2):1
Kapoor RT, Sivamani S (2021) Exploring the potential of Eucalyptus citriodora biochar against direct red 31 dye and its phytotoxicity assessment. Biomass Convers Biorefinery 1:1–12
Khan S, Malik A (2014) Environmental and health effects of textile industry wastewater. In: Environmental deterioration and human health. Springer, Dordrecht, pp 55–71
Kuppusamy S, Palanisami T, Megharaj M, Venkateswarlu K, Naidu R (2016) In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. Rev Environ Contam Toxicol 236:1–115
Missimer TM, Maliva RG (2018) Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 434:198–215
Muthuraman G (2011) Extractive removal of astacryl blue BG and astacryl golden yellow dyes from aqueous solutions by liquid–liquid extraction. Desalination 277(1–3):308–312
Muthuraman G, Palanivelu K (2006) Transport of textile dye in vegetable oils based supported liquid membrane. Dyes Pigm 70(2):99–104
Muthuraman G, Teng TT (2009a) Extraction of methyl red from industrial wastewater using xylene as an extractant. Prog Nat Sci 19(10):1215–1220
Muthuraman G, Teng TT (2009b) Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B. Desalination 249(3):1062–1066
Muthuraman G, Teng TT (2010) Solvent extraction of methyl violet with salicylic acid from aqueous acidic solutions. Desalination 263(1–3):113–117
Muthuraman G, Teng TT, Tan SH (2012) Liquid–liquid extraction of Cibacron Red FN-R by TBAB as an extractant. Desalination 284:135–141
Muthuraman G, Teng TT, Leh CP, Norli I (2009) Extraction and recovery of methylene blue from industrial wastewater using benzoic acid as an extractant. J Hazard Mater 163(1):363–369
Nascimben Santos E, László Z, Hodúr C, Arthanareeswaran G, Veréb G (2020) Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac J Chem Eng 15(5):e2533
Nithya K, Sathish A, Sivamani S (2021) In situ synthesis of mesostructured iron oxide nanoparticles embedded in L. camara: adsorption insights and modeling studies. Biomass Convers Biorefinery 1:1–12
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19(6):402–420
Shokri A, Daraei P, Zereshki S (2020) Water decolorization using waste cooking oil: an optimized green emulsion liquid membrane by RSM. J Water Process Eng 33:101021
Sivamani S, Baskar R (2018) Process design and optimization of bioethanol production from cassava bagasse using statistical design and genetic algorithm. Prep Biochem Biotechnol 48(9):834–841
Sivamani S, Baskar R, Chandrasekaran AP (2020) Response surface optimization of acid pretreatment of cassava stem for bioethanol production. Environ Prog Sustainable Energy 39(2):e13335
Soniya M, Muthuraman G (2015) Comparative study between liquid–liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J Ind Eng Chem 30:266–273
Sprakel LMJ, Schuur B (2019) Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep Purif Technol 211:935–957
Topare NS, Attar SJ, Manfe MM (2011) Sewage/wastewater treatment technologies: a review. Sci Revs Chem Commun 1(1):18–24
Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Sivamani S, Hosseini-Bandegharaei A (2021) Ecofriendly biopolymers and composites: preparation and their applications in water-treatment. Biotechnol Adv 11:07815
Vijayanand M, Varahamoorthi R, Kumaradhas P, Sivamani S (2021) Modelling and optimisation of hardness in citrate stabilised electroless nickel boron (ENi-B) coatings using back propagation neural network–Box Behnken design and simulated annealing–genetic algorithm. Trans IMF 1:1–12
Zereshki S, Daraei P, Shokri A (2018) Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J Hazard Mater 356:1–8
Acknowledgements
We would acknowledge our heartfelt thanks to the President, University of Technology and Applied Sciences, Muscat and the Management of University of Technology and Applied Sciences (Salalah College of Technology), Sultanate of Oman, for the wonderful opportunity, continuing support and encouragement by providing necessary facilities for executing the research work.
Funding
The authors received no financial support for the research, authorship, and publication of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by PK and SS; methodology was done by PK and SS; software was done by SS; validation was done by PK; formal analysis was done by PK and SS; investigation was done by PK; data curation was done by PK; writing—original draft were done by PK and SS; writing—review and editing were done by PK, SS, and KT; supervision was done by SS; project administration was done by PK and SS.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanakasabai, P., Sivamani, S. & Thirumavalavan, K. Box–Behnken design and analysis for liquid–liquid extraction of methyl red dye from its aqueous solution with benzene. Chem. Pap. 77, 7225–7235 (2023). https://doi.org/10.1007/s11696-023-03013-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03013-w