Abstract
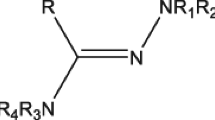
In this study, a benzidine-based azomethine derivate 2 with a proposed new mechanism and its two derivatives 4a-b have been designed, synthesized and characterized by 1H, 13C NMR, FT-IR, and HRMS spectroscopic techniques, and their anticancer properties were investigated. The target compounds 2, 4a-b were obtained with excellent yields (91% and above) by condensation of benzidine (1) with three different aldehyde derivatives (formaldehyde, benzaldehyde 3a or p-nitrobenzaldehyde 3b) in refluxing EtOH. Surprisingly, treatment of benzidine (1) with formaldehyde afforded N4,N4,N4',N4'-tetrakis(ethoxymethyl)-[1,1'-biphenyl]-4,4'-diamine (2). The anticancer properties of these benzidine derivatives 2, 4a-b against two cell lines (MDA-MB-231 human breast adenocarcinoma and DLD1 human colorectal adenocarcinoma cell lines) were investigated with a colorimetric assay using the tetrazolium salt WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salts). The obtained results showed that the benzidine-based azomethine derivatives 2, 4a-b had a significant effect on the human breast cancer cell line (MDA-MB-231). Then, molecular docking calculations were made to compare the biological activities of benzidine-based azomethine derivatives 2, 4a-b against cancer proteins. ADME/T analysis was performed to examine the drug properties of benzidine-based azomethine derivatives 2, 4a-b. The compounds 2, 4a-b are promising as potential anticancer drug candidates.
Graphical Abstract




















Similar content being viewed by others
References
Aktaş A, Tüzün B, Taşkın Kafa HA, Sayin K, Ataseven H (2020) clarification of interaction mechanism of arbidol with covid-19 and investigation of the inhibition activity analogues against covid-19. Bratisl Med J 121(10):705–711. https://doi.org/10.4149/BLL_2020_115
Aktaş A, Tüzün B, Aslan R, Sayin AH (2021) New anti-viral drugs for the treatment of COVID-19 instead of favipiravir. J Biomol Struct Dyn 39(18):7263–7273. https://doi.org/10.1080/07391102.2020.1806112
Amer YOB, El-Daghare RN, Hammouda AN, El-Ferjani RM, Elmagbari FM (2020) Synthesis and characterization of Cr(III) & Fe(II) bis(2-methoxybenzylidene)biphenyl-4,4’-diamine complexes. Open J Inorg Chem 10:6–14
Anush SM, Vishalakshi B, Kalluraya B, Manju N (2018) Synthesis of pyrazole-based schiff bases of chitosan: evaluation of antimicrobial activity. Int J Biol Macromol 119:446–452. https://doi.org/10.1016/j.ijbiomac.2018.07.129
Ashour HF, Abou-Zeid LA, El-Sayed MA, Selim KB (2020) 1,2,3-Triazole-chalcone hybrids: synthesis, in vitro cytotoxic activity and mechanistic investigation of apoptosis induction in multiple myeloma RPMI-8226. Eur J Med Chem 189:112062. https://doi.org/10.1016/j.ejmech.2020.112062
Ataseven H, Sayın K, Tüzün B, Gedikli MA (2021) Could boron compounds be effective against SARS-CoV-2? Bratisl Med J 122(10):753–758. https://doi.org/10.4149/BLL_2021_121
Bilgiçli AT, Bilgicli HG, Hepokur C, Tüzün B, Günsel A, Zengin M, Yarasir MN (2021) Synthesis of (4R)-2-(3-hydroxyphenyl) thiazolidine-4-carboxylic acid substituted phthalocyanines: anticancer activity on different cancer cell lines and molecular docking studies. Appl Organomet Chem 35:e6242. https://doi.org/10.1002/aoc.6242
Çetiner E, Sayin K, Tüzün B, Ataseven H (2021) Could boron-containing compounds (BCCs) be effective against SARS-CoV-2 as anti-viral agent? Bratisl Med J 122(4):263–269. https://doi.org/10.4149/BLL_2021_44
Chung KT (2016) Azo dyes and human health: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34(4):233–261. https://doi.org/10.1080/10590501.2016.1236602
da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CVB, de Fátima  (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2:1–8. https://doi.org/10.1016/j.jare.2010.05.004
Dhar DN, Taploo CL (1982) Schiff bases and their applications. J Sci Ind Res India 41:501–506
Erdoğan M, Yeşildağ A, Medetalibeyoğlu H, Horoz S (2023) Syntheses, DFT studies and comparatively photovoltaic applications of some pyrene-imine hybrid derivatives. Opt Mater 140:113766. https://doi.org/10.1016/j.optmat.2023.113766
Farghaly TA, Masaret GS, Muhammad ZA, Harras MF (2020) Discovery of thiazole-based-chalcones and 4-hetarylthiazoles as potent anticancer agents: synthesis, docking study and anticancer activity. Bioorg Chem 98:103761. https://doi.org/10.1016/j.bioorg.2020.103761
Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, King MC (1994) Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet 8(4):399–404. https://doi.org/10.1038/ng1294-399
Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Miki Y (1994) BRCA1 mutations in primary breast and ovarian carcinomas. Science 266(5182):120–122. https://doi.org/10.1126/science.7939630
Gedikli MA, Tuzun B, Aktas A, Sayin K, Ataseven H (2021a) Are clarithromycin, azithromycin and their analogues effective in the treatment of COVID19? Bratisl Med J 122(2):101–110. https://doi.org/10.4149/BLL_2021_015
Gedikli MA, Tuzun B, Sayin K, Ataseven H (2021b) Determination of inhibitor activity of drugs against the COVID-19. Bratisl Med J 122(7):497–506. https://doi.org/10.4149/BLL_2021_81
Gezegen H, Gürdere MB, Dinçer A, Özbek O, Koçyiğit ÜM, Taslimi P, Ceylan M (2021) Synthesis, molecular docking, and biological activities of new cyanopyridine derivatives containing phenylurea. Arch Pharm 354(4):2000334. https://doi.org/10.1002/ardp.202000334
Göbel A, Leibeling G, Rudolph M, Imhof W (2003) Tuning the electronic communication between iron carbonyl fragments coordinated to bis-imine ligands by variation of the bridging unit. Organometallics 22:759–768. https://doi.org/10.1021/om020850i
Gürdere MB, Budak Y, Kocyigit UM, Taslimi P, Tüzün B, Ceylan M (2021) ADME properties, bioactivity and molecular docking studies of 4-amino-chalcone derivatives: new analogues for the treatment of alzheimer, glaucoma and epileptic diseases. Silico Pharmacol 9(34):1–11. https://doi.org/10.1007/s40203-021-00094-x
Haley TJ (1975) Benzidine revisited: a review of the literature and problems associated with the use of benzidine and its congeners. Clin Toxicol 8:13–42. https://doi.org/10.3109/15563657508988044
Harpstrite SE, Collins SD, Oksman A, Goldberg DE, Sharma V (2008) Synthesis, characterization, and antimalarial activity of novel schiff-base-phenol and naphthalene-amine ligands. Med Chem 4:392–395. https://doi.org/10.2174/157340608784872280
Iqbal A, Siddiqui HL, Ashraf CM, Bukhari MH, Akram CM (2007) Synthesis, spectroscopic and cytotoxic studies of biologically active new schiff bases derived from p-nitrobenzaldehyde. Chem Pharm Bull 55:1070–1072. https://doi.org/10.1248/cpb.55.1070
Joseph J, Nagashri K, Rani GAB (2013) Synthesis, characterization and antimicrobial activities of copper complexes derived from 4-aminoantipyrine derivatives. J Saudi Chem Soc 17:285–294. https://doi.org/10.1016/j.jscs.2011.04.007
Kennedy NJ, Davis RJ (2003) Role of JNK in tumor development. Cell Cycle (georgetown, Tex) 2(3):199–201
Krzysztof Sztanke AM, Osinka A, Sztanke M (2013) An insight into synthetic schiff bases revealing antiproliferative activities in vitro. Bioorg Med Chem 21:3648–3666. https://doi.org/10.1016/j.bmc.2013.04.037
Lapasam A, Dkhar L, Joshi N, Poluri KM, Kollipara MR (2019) Antimicrobial selectivity of ruthenium, rhodium, and iridium half sandwich complexes containing phenyl hydrazone Schiff base ligands towards B. thuringiensis and P. aeruginosa bacteria. Inorg Chim Acta 484:255–263. https://doi.org/10.1016/j.ica.2018.09.067
Marusiak AA, Stephenson NL, Baik H, Trotter EW, Li Y, Blyth K, Brognard J (2016) Recurrent MLK4 loss-of-function mutations suppress JNK signaling to promote colon tumorigenesis. Cancer Res 76(3):724–735. https://doi.org/10.1158/0008-5472.CAN-15-0701-T
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Ding W (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266(5182):66–71. https://doi.org/10.1126/science.7545954
Miri R, Razzaghi-asl N, Mohammadi MK (2013) QM study and conformational analysis of an isatin schiff base as a potential cytotoxic agent. J Mol Model 19:727–735. https://doi.org/10.1007/s00894-012-1586-x
Modi JD, Sabnis SS, Deliwala CV (1970) Potential anticancer agents. III. Schiff bases from benzaldehyde nitrogen mustards and aminophenylthiazoles. J Med Chem 13(5):935–1941. https://doi.org/10.1021/jm00299a031
Muskinja JM, Burmudzija AZ, Baskic DD, Popovic SL, Todorovic DV, Zaric MM, Ratkovic ZR (2019) Synthesis and anticancer activity of chalcone analogues with sulfonyl groups. Med Chem Res 28:279–291. https://doi.org/10.1007/s00044-018-02283-4
Mutahir S, Khan MA, Khan IU, Yar M, Ashraf M, Tariq S, Ye RL, Zhou BJ (2017) Organocatalyzed and mechanochemical solvent-free synthesis of novel and functionalized bis-biphenyl substituted thiazolidinones as potent tyrosinase inhibitors: SAR and molecular modeling studies. Eur J Med Chem 134:406–414. https://doi.org/10.1016/j.ejmech.2017.04.021
Ngameni B, Cedric K, Mbaveng AT, Erdogan M, Simo I, Kuete V, Dastan A (2021) Design, synthesis, characterization, and anticancer activity of a novel series of O-substituted chalcone derivatives. Bioorg Med Chem Lett 35:127827. https://doi.org/10.1016/j.bmcl.2021.127827
Pawar S, Kumar A (2002) Issues in the formulation of drugs for oral use in children: role of excipients. Paediatr Drugs 4(6):371–379. https://doi.org/10.2165/00128072-200204060-00004
Petrus ML, Bouwer RKM, Lafont U, Athanasopoulos S, Greenham NC, Dingemans TJ (2014) Small-molecule azomethines: organic photovoltaics via Schiff base condensation chemistry. J Mater Chem A 2:9474–9477. https://doi.org/10.1039/C4TA01629G
Prival MJ, Bell SJ, Mitchell VD, Peiperl MD, Vaughan VL (1984) Mutagenicity of benzidine and benzidine-congener dyes and selected monoazo dyes in a modified salmonella assay. Mutat Res 136:33–47. https://doi.org/10.1016/0165-1218(84)90132-0
Qin WL, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18(10):12264–12289. https://doi.org/10.3390/molecules181012264
Rathelot P, Vanelle P, Gasquet M, Delmas F, Crozet MP, Timondavid P, Maldonado J (1995) Synthesis of novel functionalized 5-nitroisoquinolines and evaluation of in vitro antimalarial activity. Eur J Med Chem 30:503–508. https://doi.org/10.1016/0223-5234(96)88261-4
Riaz S, Iqbal M, Ullah R, Zahra R, Chotana GA, Faisal A, Saleem RSZ (2019) Synthesis and evaluation of novel α-substituted chalcones with potent anticancer activities and ability to overcome multidrug resistance. Bioorg Chem 87:123–135. https://doi.org/10.1016/j.bioorg.2019.03.014
Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol 5:12. https://doi.org/10.1186/1472-6750-5-12
Schrodinger L (2019) Small-molecule drug discovery suite 2019–4. Schrödinger, New York
Schrödinger Release 2019-4 (2019a) Protein preparation wizard Epik. Schrödinger LLC, New York, NY
Schrödinger Release 2019-4 (2019b) LigPrep. Schrödinger LLC, New York, NY
Schrödinger Release 2020-1 (2020) QikProp. Schrödinger LLC, New York, NY
Šuleková M, Smrčová M, Hudák A, Heželová M, Fedorová M (2017) Organic colouring agents in the pharmaceutical industry. Folia Vet 61(3):32–46. https://doi.org/10.1515/fv-2017-0025
Turhan F, Pak F, Yeşildağ A, Kudaş ZE, D, (2012) Electrochemical synthesis and characterization of poly(9-benzylfluorene). Polym Bull 68:1677–1687. https://doi.org/10.1007/s00289-011-0667-9
Türkan F, Taslimi P, Cabir B, Ağırtaş MS, Erden Y, Celebioglu HU, Gulcin I (2022) Co and Zn metal phthalocyanines with bulky substituents: anticancer, antibacterial activities and their inhibitory effects on some metabolic enzymes with molecular docking studies. Polycycl Aromat Compd 42(7):4475–4486. https://doi.org/10.1080/10406638.2021.1893194
Tüzün B (2020) Examination of anti-oxidant properties and molecular docking parameters of some compounds in human body. Turk Comput Theor Chem 4(2):76–87. https://doi.org/10.33435/tcandtc.781008
Tüzün B, Nasibova T, Garaev E, Sayın K, Ataseven H (2021) Could alkaloids be effective in the treatment of COVID-19? Bratisl Med J 122(9):670–679. https://doi.org/10.4149/BLL_2021_108
Uddin N, Rashid F, Ali S, Tirmizi SA, Ahmad I, Zaib S, Zubair M, Diaconescu PL, Tahir MN, Iqbal J, Haider A (2020) Synthesis, characterization, and anticancer activity of Schiff bases. J Biomol Struct Dyn 38:3246–3259. https://doi.org/10.1080/07391102.2019.1654924
Venugopala KN, Jayashree BS (2003) Synthesis and characterization of carboxamides of 2′-amino-4′-(6-bromo-3-coumarinyl) thiazole for their analgesic and anti-inflammatory activity. Indian J Heterocycl Chem 12:307–310
Wang GB, Chang JC (1994) Synthesis and characterization of amino acid schiff base complexes of nickel(II). Synth React Inorg Met 24:1091–1097. https://doi.org/10.1080/00945719408001385
Whitmarsh AJ, Davis RJ (2007) Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene 26(22):3172–3184. https://doi.org/10.1038/sj.onc.1210410
Williams RS, Green R, Glover JM (2001) Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat Struct Biol 8(10):838–842. https://doi.org/10.1038/nsb1001-838
**n Y, Yuan JY (2012) Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym Chem 3(11):3045–3055. https://doi.org/10.1039/C2PY20290E
Yeşildağ A, Erdoğan M, Sevgili Ö, Çaldıran Z, Orak I (2021) Optical and electrical properties of pyrene-imine organic interface layer based on p-Si. J Electron Mater 50:6448–6458. https://doi.org/10.1007/s11664-021-09178-y
Yeşildag A, Erdogan M, Medetalibeyoglu H, Horoz S (2022) Synthesis of benzidine-based conjugated organic materials bearing donor-acceptor groups: DFT studies and photovoltaic applications. J Mol Struct 1251:131939. https://doi.org/10.1016/j.molstruc.2021.131939
Funding
This research has been supported by Kafkas University Scientific Research Projects Coordination Unit. Project Number 2021-FM-66 Year 2021.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erdoğan, M., Yeşildağ, A., Yıldız, B. et al. Synthesis and characterization of some benzidine-based azomethine derivatives with molecular docking studies and anticancer activities. Chem. Pap. 77, 6829–6847 (2023). https://doi.org/10.1007/s11696-023-02981-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02981-3