Abstract
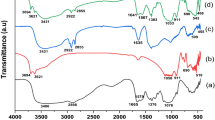
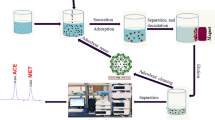
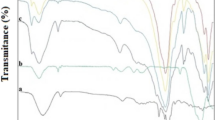
In this study, the magnetic biosorbent was synthesized from kiwi peel and applied to eliminate Sitagliptin (STG) and Fampridine (FMP) from aqueous media using high-performance liquid chromatography (HPLC). The maximum removal percentage was found to be 69.04% and 63.76% for STG and FMP, respectively. The characterization of adsorbent was evaluated using a scanning electron microscope (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction analysis (XRD), and vibrating-sample magnetometer (VSM). The optimization of variables, including pH, adsorbent dosage, and contact time, influencing the peak area were investigated via the central composite design (CCD). The highest peak area of the STG (actual: 2170.1, predicted: 2170.21) was obtained at a pH of 7.8, an adsorbent dosage of 15.5 mg, and a contact time of 35.5 min. Also, the maximum peak area of FMP (actual: 1932.5, predicted: 1901.11) was related to the pH = 7.8, adsorbent dosage of 11.8 mg, and contact time of 33.5 min. The significance and adequacy of the model and experimental variables were assessed using analysis of variance (ANOVA). The results indicated that the quadratic model is acceptable for the prediction of peak areas of STG and FMP. The magnetic kiwi peel as a simple and low-cost adsorbent with easy fabrication was found to be efficient for STG and FMP removal from the aqueous environments.













Similar content being viewed by others
References
Abbaszadeh S, Rashidi Nodeh H, Rafidah Wan Alwi S (2017) Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination. Pure Appl Chem 90:1
Abolhasani S, Ahmadpour A, Rohani Bastami T, Yaqubzadeh A (2019) Facile synthesis of mesoporous carbon aerogel for the removal of ibuprofen from aqueous solution by central composite experimental design (CCD). J Mol Liq 281:261–268
Achanti S, Rajeswari Katta R (2016) High-throughput liquid chromatography tandem mass spectrometry method for simultaneous determination of fampridine, paroxetine, and quinidine in rat plasma: application to in vivo perfusion study. J Food Drug Anal 24:866–875
Akpomie KG, Conradie J (2020) Efficient synthesis of magnetic nanoparticle-Musa acuminata peel composite for the adsorption of anionic dye. Arab J Chem 13:7115–7131
Almomani FA, Shawaqfah M, Bhosale RR, Kumar A (2016) Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ Prog Sustainable Energy 35:982–995
Angeles LF, Mullen RA, Huang IJ, Wilson C, Khunjar W, Sirotkin HI, McElroy AE, Aga DS (2020) Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems. Environ Sci: Water Res Technol 6:62
Bankole DT, Oluyori AP, Inyinbor AA (2023) The removal of pharmaceutical pollutants from aqueous solution by Agro-waste. Arab J Chem 16:104699
Baresel C, Harding M, Fang J (2019) Ultrafiltration/granulated active carbon-biofilter: efficient removal of a broad range of micropollutants. Appl Sci 9:710
Bayuo J, Abdullai Abukari M, Bayetimani Pelig-Ba K (2020) Optimization using central composite design (CCD) of response surface methodology (RSM) for biosorption of hexavalent chromium from aqueous media. Appl Water Sci 10:135
Bhattacharyya S, Das P, Datta S (2019) Removal of Ranitidine from PharmaceuticalWasteWater Using Activated Carbon (AC) Prepared fromWaste Lemon Peel, Waste Water Recycling and Management. pp 123–141.
Castro D, Rosas-Laverde NM, Aldás MB, Almeida-Naranjo CE, Guerrero VH, Pruna AI (2021) Chemical modification of agro-industrial waste-based bioadsorbents for enhanced removal of Zn (II) ions from aqueous solutions. Materials 14(9):2134
Chang Lin C, Yen Lee C (2020) Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Mater Chem Phys 240:122049
Dhiman N, Sharma N (2019) Removal of pharmaceutical drugs from binary mixtures by use of ZnO nanoparticles. Environ Technol Innov 15:100392
Elshafei AM, Othman AM, Elsayed MA, Al-Balakocy NG, Hassan MM (2021) Green synthesis of silver nanoparticles using Aspergillus oryzae NRRL447 exogenous proteins: optimization via central composite design, characterization and biological applications, environmental nanotechnology. Monitoring & Management 16:100553
Farhadi SH, Sohrabi MR, Motiee F, Davallo M (2021) Organophosphorus diazinon pesticide removing from aqueous solution by zero-valent iron supported on biopolymer chitosan: Rsm optimization methodology. J Polym Environ 29:103–120
Gomes D, Cardoso M, Martins RC, Quinta-Ferreira RM, Gando-Ferreira LM (2020) Removal of a mixture of pharmaceuticals sulfamethoxazole and diclofenac from water streams by a polyamide nanofiltration membrane. Water Sci Technol 81:732–743
Gou X, Zhang P, Song Y, Qian F, Yu H, Zeng G (2017) Novel insights into the coagulation process for pharmaceutical wastewater treatment with fluorescence EEMs-PARAFAC. Water Sci Technol 76:3246–3257
Gubitosa J, Rizzi V, Fini P, Nuzzo S, Cosma P (2022a) Regenerable kiwi peels as an adsorbent to remove and reuse the emerging pollutant propranolol fromwater. Processes 10:1417
Gubitosa J, Rizzi V, Cignolo D, Fini P, Fanelli F, Cosma P (2022b) From agricultural wastes to a resource: kiwi Peels, as long-lasting, recyclable adsorbent, to remove emerging pollutants from water the case of ciprofloxacin removal. Sustain Chem Pharmacy 29:100749
Gumieniczek A, Berecka A, Mroczek T, Wojtanowski K, Dabrowska K, Stepie´n K, (2019) Determination of chemical stability of sitagliptin by LC-UV, LC-MS and FT-IR methods. J Pharm Biomed Anal 164:789–807
Hermes N, Jewell KS, Falas P, Lutze HV, Wick A, Ternes TA (2020) Ozonation of sitagliptin: removal kinetics and elucidation of oxidative transformation products. Environ Sci Technol 54:10588–10598
Jiang R, Tian J, Zheng H, Qi J, Sun S, Li X (2015) A novel magnetic adsorbent based on waste litchi peels for removing Pb(II) from aqueous solution. J Environ Manage 155:24–30
Kalantary RR, Azari A, Esrafili A, Yaghmaeian K, Moradi M, Sharafi K (2016) The survey of Malathion removal using magnetic graphene oxide nanocomposite as a novel adsorbent: thermodynamics, isotherms, and kinetic study. Desalin Water Treat 57:28460–28473
Kanakaraju D, Glass BD, Oelgemoller M (2018) Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J Environ Manage 219:189–207
Kaur M, Kumari S, Sharma P (2020) Removal of Pb (II) from aqueous solution using nanoadsorbent of Oryza sativa husk: Isotherm, kinetic and thermodynamic studies. Biotechnol Reports 25:e00410
Kit Loh GO, Yii Ling Wong E, Tze Fung Tan Y, Lin Lee Y, Hui Pang L, Chien Chin M, Damenthi N, Khiang Peh K (2020) Simple and rapid LC-MS/MS method for determination of sitagliptin in human plasma and application to bioequivalence study. J Chromatogr B 1159:122337
Kooijman G, de Kreuk MK, Houtman C, Van Lier JB (2020) Perspectives of coagulation/flocculation for the removal of pharmaceuticals from domestic wastewater: a critical view at experimental procedures. J Water Process Eng 34:101161
Krakko D, Illes A, Domjan A, Demeter A, Dobe S, Zaray G (2022) UV and (V)UV irradiation of sitagliptin in ultrapure water and WWTP effluent: Kinetics, transformation products and degradation pathway. Chemosphere 288:132393
Kyzasa GZ, Deliyanni EA (2015) Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem Eng Res Des 97:135–144
Liakos EV, Lazaridou M, Michailidou G, Koumentakou I, Lambropoulou DA, Bikiaris DN, Kyzas GZ (2021) Chitosan adsorbent derivatives for pharmaceuticals removal from effluents: a review. Macromol 1:130–154
Liu M, Zhang X, Li Z, Qu L, Han R (2020) Fabrication of zirconium (IV)-loaded chitosan/Fe3O4/graphene oxide for efficient removal of alizarin red from aqueous solution. Carbohyd Polym 248:116792
Mahmoud ME, Moneim El-Ghanam A, Saad SR, Mohamed HA, R, (2020) Promoted removal of metformin hydrochloride anti-diabetic drug from water by fabricated and modified nanobiochar from artichoke leaves. Sustainable Chem Pharmacy 18:100336
Mallampati R, Xuanjun L, Adin A, Valiyaveettil S (2015) Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water. ACS Sustainable Chemistry & Engineering 3:1117–1124
Mohd Zahri NA, Md Jamil SNA, Abdullah LC, Jia Huey S, Nourouzi Mobarekeh M, Mohd Rapeia NS, Shean Yaw TC (2021) Central composite design of heavy metal removal using polymer adsorbent. J Appl Water Eng Res 9(2):133–146
Mondal S, Sinha K, Aikat K, Halder G (2015) Adsorption thermodynamics and kinetics of ranitidine hydrochloride onto superheated steam activated carbon derived from mung bean husk. J Environ Chem Eng 3:187–195
Mrozik W, Stefanska J (2014) Adsorption and biodegradation of antidiabetic pharmaceuticals in soils. Chemosphere 95:281–288
Pashazadeh-Panahi P, Hasanzadeh M (2020) efficient removal of digoxin from aqueous solution using magnetic nanocomposite (Fe3O4–GO–SO3H) as an advanced nano-absorbent. Nanocomposites 6:66–75
Pate M, Kumar R, Pittman C, Mohan D (2021) Ciprofloxacin and acetaminophen sorption onto banana peel biochars: environmental and process parameter influences. Environ Res 201:111218
Pathak PD, Mandavgane SA, Kulkarni BD (2015) Fruit peel waste as a novel low-cost bio adsorbent. Rev Chem Eng 31:361–381
Patil CS, Gunjal DB, Naik VM, Harale NS, Jagadale SD, Kadam AN, Patil PS, Kolekar GB, Gore AH (2019) Waste tea residue as a low cost adsorbent for removal of Hydralazine hydrochloride pharmaceutical pollutant from aqueous media: an environmental remediation. J Clean Prod 206:407–418
Rajapaksha AU, Vithanage M, Zhang M, Ahmad M, Mohan D, Chang SX, Sik Ok Y (2014) Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Biores Technol 166:303–308
Rani M, Shanker U (2018) Effective adsorption and enhanced degradation of various pesticides from aqueous solution by Prussian blue nanorods. J Environ Chem Eng 6:1512–1521
Rao Amarachinta P, Sharma G, Samed N, Kumar Chettupalli A, Alle M, Kim JC (2021) Central composite design for the development of carvedilol-loaded transdermal ethosomal hydrogel for extended and enhanced anti-hypertensive effect. J Nanobiotechnol 19:100
Sanz V, López-Hortas L, Torres MD, Domínguez H (2021) Trends in kiwifruit and byproducts valorization. Trends Food Sci Technol 107:401–414
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238
Singh AK, Ketan K, Singh JK (2017) Simple and green fabrication of recyclable magnetic highly hydrophobic sorbents derived from waste orange peels for removal of oil and organic solvents from water surface. J Environ Chem Eng 5:5250–5259
Suneetha A, Raja Rajeswari K (2016) A high throughput flow gradient LC–MS/MS method for simultaneous determination of fingolimod, fampridine and prednisone in rat plasma, application to in vivo perfusion study. J Pharm Biomed Anal 120:10–18
Swarnalakshmi KS, Chinnaiyan P, Nivetha S, Nair AS (2018) Use of rice husk ash as an adsorbent to remove contaminants in water and comparison with advanced oxidation process – a study. Materials Today: Proceedings 5:24248–24257
Weller D, Lörincz L, Sutter T, Reuter K, Linnebank M, Weller M, Zörner B, Filli L (2020) Fampridine-induced changes in walking kinetics are associated with clinical improvements in patients with multiple sclerosis. J Neurol Sci 416:116978
Weng X, Ma L, Guo M, Su Y, Dharmarajan R, Chen Z (2018) Removal of doxorubicin hydrochloride using Fe3O4 nanoparticles synthesized by euphorbia cochinchinensis extract. Chem Eng J 353:482–489
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslanzadeh, F., Konoz, E., Niazi, A. et al. Simple and rapid synthesis of magnetic kiwifruit for the removal of sitagliptin and fampridine residues from aqueous media using HPLC method: optimization via central composite experimental design. Chem. Pap. 77, 5097–5114 (2023). https://doi.org/10.1007/s11696-023-02847-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02847-8