Abstract
Background
The resolution of type 2 diabetes mellitus is an additional outcome of Roux-en-Y gastric bypass (RYGB) surgery. The general objective was to explore whether RYGB could reduce beta cells apoptosis and what roles adiponectin played in downregulating hyperglycemia after RYGB.
Methods
Twenty Goto-Kakizaki (GK) rats were allocated in RYGB group (ten) and GK group (ten), and ten Wistar (WS) rats were allocated in WS group. RYGB was performed in RYGB group and sham operation in the GK and WS groups. Fasting plasma glucose, body weight, food intake per 100 g body weight, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), C peptide, and adiponectin were measured pre- and postoperatively. Terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate nick end-labeling and transmission electron microscopy were performed to detect apoptosis of pancreatic beta cells. Data were analyzed by analysis of variance, Student t test, and post hoc comparisons (Tukey’s test).
Results
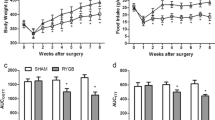
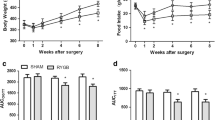
Animals in WS group had significant higher postprandial insulin, C peptide, and adiponectin concentrations compared to RYGB and GK groups preoperatively. Body weight and food intake in RYGB group significantly decreased compared to WS and GK groups postoperatively. Postprandial insulin, C peptide, and adiponectin concentrations significantly increased, while fasting plasma glucose and HOMA-IR values decreased in RYGB group compared to GK group postoperatively. More apoptotic beta cells were detected in GK group than RYGB and WS groups postoperatively.
Conclusions
RYGB could increase postprandial insulin and reduce pancreatic islet apoptosis. Adiponectin played a key role in regulating plasma glucose and reducing pancreatic islet apoptosis after RYGB.



Similar content being viewed by others
References
Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101.
Donnelly R, Wang B, Qu X. Type 2 diabetes in China: partnerships in education and research to evaluate new antidiabetic treatments. Br J Clin Pharmacol. 2006;61:702–5.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–11.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81.
Brolin RE. Bariatric surgery and long-term control of morbid obesity. Jama. 2002;288:2793–6.
Chelikani PK, Shah IH, Taqi E, et al. Comparison of the effects of Roux-en-Y gastric bypass and ileal transposition surgeries on food intake, body weight, and circulating peptide YY concentrations in rats. Obes Surg. 2010;20:1281–8.
Fruhbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–9.
Spector D, Shikora S. Neuro-modulation and bariatric surgery for type 2 diabetes mellitus. Int J Clin Pract Suppl. 2010;166:53–8.
Pournaras DJ, Osborne A, Hawkins SC, et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56–60.
Luo N, Liu J, Chung BH, et al. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59:791–9.
Gu W, Li X, Liu C, et al. Globular adiponectin augments insulin secretion from pancreatic islet beta cells at high glucose concentrations. Endocr. 2006;30:217–21.
Zhuo Q, Wang ZQ, Fu P, et al. Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomed Environ Sci. 2010;23:53–61.
Yamashita T, Matsuda M, Nishimoto O, et al. Combination of serum adiponectin level and metabolic syndrome is closely associated with coronary artery disease in Japanese subjects with good glycemic control. Intern Med. 2010;49:721–7.
Bik W, Baranowska-Bik A, Wolinska-Witort E, et al. The relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese women. Neuro Endocrinol Lett. 2006;27:493–500.
Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes to ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8.
Berg AH, Combs TP, Du X, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53.
Meguid MM, Ramos EJ, Suzuki S, et al. A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg. 2004;8:621–30.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Shim WS, Kim SK, Kim HJ, et al. Decrement of postprandial insulin secretion determines the progressive nature of type-2 diabetes. Eur J Endocrinol. 2006;155:615–22.
Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10.
Huypens PR. Leptin and adiponectin regulate compensatory beta cell growth in accordance to overweight. Med Hypotheses. 2007;68:1134–7.
Wijesekara N, Krishnamurthy M, Bhattacharjee A, et al. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623–31.
Engl J, Bobbert T, Ciardi C, et al. Effects of pronounced weight loss on adiponectin oligomer composition and metabolic parameters. Obesity (Silver Spring). 2007;15:1172–8.
Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–40.
Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62.
Okamoto M, Ohara-Imaizumi M, Kubota N, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–35.
Mao X, Kikani CK, Riojas RA, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–23.
Yoon MJ, Lee GY, Chung JJ, et al. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70.
Kamon J, Yamauchi T, Terauchi Y, et al. The mechanisms by which PPARgamma and adiponectin regulate glucose and lipid metabolism. Nippon Yakurigaku Zasshi. 2003;122:294–300.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Mithieux G, Andreelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2009;12:419–23.
Acknowledgment
The authors would like to thank all participants for their cooperation and particularly Dr. Yu Yun for designing and revising the papers. This study was supported by Foundation from National Natural Science Foundation of China (81000158), Natural Science Foundation of LiaoNing Province (20092128), and Educational Foundation of LiaoNing Province (2009a717).
Potential Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, F., Wang, Y., Zhou, Y. et al. Adiponectin Downregulates Hyperglycemia and Reduces Pancreatic Islet Apoptosis After Roux-En-Y Gastric Bypass Surgery. OBES SURG 21, 768–773 (2011). https://doi.org/10.1007/s11695-011-0357-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0357-6