Abstract
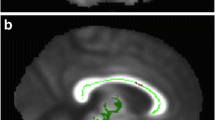
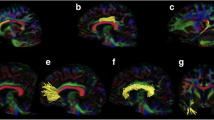
This study aimed to identify damaged segments of brain white matter fiber tracts in patients with systemic lupus erythematosus (SLE) using diffusion tensor imaging (DTI)-based automated fiber quantification (AFQ), and analyze their relationship with cognitive impairment. Clinical and imaging data for 39 female patients with SLE and for 44 female healthy controls (HCs) were collected. AFQ was used to track whole-brain white matter tracts in each participant, and each tract was segmented into 100 equally spaced nodes. DTI metrics including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were calculated at each node. Correlations were also explored between DTI metrics in the damaged segments of white matter fiber tracts and neuropsychological test scores of patients with SLE. Compared with HCs, SLE patients exhibited significantly lower FA values, and significantly higher MD, AD, RD values in many white matter tracts (all P < 0.05, false discovery rate-corrected). FA values in nodes 97–100 of the left inferior fronto-occipital fasciculus (IFOF) positively correlated with the mini-mental state examination score. AFQ enables precise and accurate identification of damage to white matter fiber tracts in brains of patients with SLE. FA values in the left IFOF correlate with cognitive impairment in SLE.


Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Conti, F., Alessandri, C., Perricone, C., Scrivo, R., Rezai, S., Ceccarelli, F., Spinelli, F. R., Ortona, E., Marianetti, M., Mina, C., & Valesini, G. (2012). Neurocognitive dysfunction in systemic lupus erythematosus: Association with antiphospholipid antibodies, disease activity and chronic damage. PloS One, 7(3), e33824. https://doi.org/10.1371/journal.pone.0033824.
Corrêa, D. G., Zimmermann, N., Pereira, D. B., Doring, T. M., Netto, T. M., Ventura, N., Fonseca, R. P., & Gasparetto, E. L. (2016). Evaluation of white matter integrity in systemic lupus erythematosus by diffusion tensor magnetic resonance imaging: A study using tract-based spatial statistics. Neuroradiology, 58(8), 819–825. https://doi.org/10.1007/s00234-016-1688-8.
Gulati, G., Jones, J. T., Lee, G., Altaye, M., Beebe, D. W., Meyers-Eaton, J., Wiley, K., Brunner, H. I., & DiFrancesco, M. W. (2017). Altered blood-brain barrier permeability in patients with systemic lupus erythematosus: A Novel Imaging Approach. Arthritis care & Research, 69(2), 299–305. https://doi.org/10.1002/acr.22923.
Ho, R. C., Thiaghu, C., Ong, H., Lu, Y., Ho, C. S., Tam, W. W., & Zhang, M. W. (2016). A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmunity Reviews, 15(2), 124–138. https://doi.org/10.1016/j.autrev.2015.10.003.
Hochberg, M. C. (1997). Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism, 40(9), 1725. https://doi.org/10.1002/art.1780400928.
Hoy, A. R., Ly, M., Carlsson, C. M., Okonkwo, O. C., Zetterberg, H., Blennow, K., Sager, M. A., Asthana, S., Johnson, S. C., Alexander, A. L., & Bendlin, B. B. (2017). Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PloS One, 12(3), e0173982. https://doi.org/10.1371/journal.pone.0173982.
Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., Calabresi, P. A., Pekar, J. J., van Zijl, P. C., & Mori, S. (2008). Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage, 39(1), 336–347. https://doi.org/10.1016/j.neuroimage.2007.07.053.
Huang, L., Chen, X., Sun, W., Chen, H., Ye, Q., Yang, D., Li, M., Luo, C., Ma, J., Shao, P., Xu, H., Zhang, B., Zhu, X., & Xu, Y. (2021). Early segmental White Matter Fascicle Microstructural damage predicts the corresponding cognitive domain impairment in Cerebral Small Vessel Disease patients by Automated Fiber quantification. Frontiers in Aging Neuroscience, 12, 598242. https://doi.org/10.3389/fnagi.2020.598242.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). FSL NeuroImage, 62(2), 782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015.
Jiang, Y., Liu, Y., Gao, B., Che, Y., Lin, L., Jiang, J., Chang, P., Song, Q., Wang, N., Wang, W., & Miao, Y. (2021). Segmental abnormalities of White Matter Microstructure in End-Stage Renal Disease patients: An automated Fiber quantification Tractography Study. Frontiers in Neuroscience, 15, 765677. https://doi.org/10.3389/fnins.2021.765677.
Jung, R. E., Chavez, R. S., Flores, R. A., Qualls, C., Sibbitt, W. L. Jr., & Roldan, C. A. (2012). White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PloS One, 7(1), e28373. https://doi.org/10.1371/journal.pone.0028373.
Kivity, S., Katzav, A., Arango, M. T., Landau-Rabi, M., Zafrir, Y., Agmon-Levin, N., Blank, M., Anaya, J. M., Mozes, E., Chapman, J., & Shoenfeld, Y. (2013). 16/6-idiotype expressing antibodies induce brain inflammation and cognitive impairment in mice: The mosaic of central nervous system involvement in lupus. BMC Medicine, 11, 90. https://doi.org/10.1186/1741-7015-11-90.
Kozora, E., Uluğ, A. M., Erkan, D., Vo, A., Filley, C. M., Ramon, G., Burleson, A., Zimmerman, R., & Lockshin, M. D. (2016). Functional Magnetic Resonance Imaging of Working Memory and executive dysfunction in systemic lupus erythematosus and antiphospholipid antibody-positive patients. Arthritis care & Research, 68(11), 1655–1663. https://doi.org/10.1002/acr.22873.
Li, S. G., Wang, Y. Q., Huang, X. Q., Lv, S., Zhang, W., Qiu, C. J., & Gong, Q. Y. (2014). Whole brain diffusion tensor imaging in diagnosing social anxiety disorder based on support vector machine. Zhong Hua Fang She Xue Za Zhi, 48(8), 636–640.
Mackay, M., Vo, A., Tang, C. C., Small, M., Anderson, E. W., Ploran, E. J., Storbeck, J., Bascetta, B., Kang, S., Aranow, C., Sartori, C., Watson, P., Volpe, B. T., Diamond, B., & Eidelberg, D. (2019). Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight, 4(1), e124002. https://doi.org/10.1172/jci.insight.124002.
Muscal, E., & Brey, R. L. (2010). Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurologic Clinics, 28(1), 61–73. https://doi.org/10.1016/j.ncl.2009.09.004.
Surbeck, W., Hänggi, J., Scholtes, F., Viher, P. V., Schmidt, A., Stegmayer, K., Studerus, E., Lang, U. E., Riecher-Rössler, A., Strik, W., Seifritz, E., Borgwardt, S., Quednow, B. B., & Walther, S. (2020). Anatomical integrity within the inferior fronto-occipital fasciculus and semantic processing deficits in schizophrenia spectrum disorders. Schizophrenia Research, 218, 267–275. https://doi.org/10.1016/j.schres.2019.12.025.
Wakana, S., Caprihan, A., Panzenboeck, M. M., Fallon, J. H., Perry, M., Gollub, R. L., Hua, K., Zhang, J., Jiang, H., Dubey, P., Blitz, A., van Zijl, P., & Mori, S. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage, 36(3), 630–644. https://doi.org/10.1016/j.neuroimage.2007.02.049.
Wang, J., Ma, L., Liu, G., Bai, W., Ai, K., Zhang, P., Hu, W., & Zhang, J. (2022). Tractography in type 2 diabetes Mellitus with subjective memory complaints: A diffusion Tensor Imaging Study. Frontiers in Neuroscience, 15, 800420. https://doi.org/10.3389/fnins.2021.800420.
Wang, L., Zheng, G., Jia, X., Zhang, X., & Chen, Y. (2023). Application Study of Brain Structure and Functional Magnetic Resonance Imaging in Patients with Systemic Lupus Erythematosus and Cognitive Dysfunction. Alternative therapies in health and medicine, AT9171. Advance online publication.
Whittaker, H. T., Zhu, S., Di Curzio, D. L., Buist, R., Li, X. M., Noy, S., Wiseman, F. K., Thiessen, J. D., & Martin, M. (2018). T1, diffusion tensor, and quantitative magnetization transfer imaging of the hippocampus in an Alzheimer’s disease mouse model. Magnetic Resonance Imaging, 50, 26–37. https://doi.org/10.1016/j.mri.2018.03.010.
Yan, Z., Wang, X., Zhu, Q., Shi, Z., Chen, X., Han, Y., Zheng, Q., Wei, Y., Wang, J., & Li, Y. (2022). Alterations in White Matter Fiber tracts characterized by automated Fiber-tract quantification and their correlations with cognitive impairment in Neuromyelitis Optica Spectrum Disorder patients. Frontiers in Neuroscience, 16, 904309. https://doi.org/10.3389/fnins.2022.904309.
Yeatman, J. D., Dougherty, R. F., Myall, N. J., Wandell, B. A., & Feldman, H. M. (2012). Tract profiles of white matter properties: Automating fiber-tract quantification. PloS One, 7(11), e49790. https://doi.org/10.1371/journal.pone.0049790.
Yu, H., Qiu, X., Zhang, Y. Q., Deng, Y., He, M. Y., Zhao, Y. T., & Zhai, Z. H. (2019). Abnormal amplitude of low frequency fluctuation and functional connectivity in non-neuropsychiatric systemic lupus erythematosus: A resting-state fMRI study. Neuroradiology, 61(3), 331–340. https://doi.org/10.1007/s00234-018-2138-6.
Yu, C. Y., Qiu, W. C., Sun, J. T., Gao, Y., & Wang, X. S. (2021). Study on the changes of white matter fiber integrity in children with CAE based on AFQ. Nan **g Yi Ke Da Xue Bao Zi Ran Ke Xue Ban, 41(12), 1767–1773.
Yu, B., Ding, Z., Wang, L., Feng, Q., Fan, Y., Xu, X., & Liao, Z. (2022). Application of Diffusion Tensor Imaging based on automatic Fiber quantification in Alzheimer’s Disease. Current Alzheimer Research, 19(6), 469–478. https://doi.org/10.2174/1567205019666220718142130.
Zhang, X., Sun, Y., Li, W., Liu, B., Wu, W., Zhao, H., Liu, R., Zhang, Y., Yin, Z., Yu, T., Qing, Z., Zhu, B., Xu, Y., Nedelska, Z., Hort, J., & Zhang, B. (2019). Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer’s disease. NeuroImage Clinical, 22, 101723. https://doi.org/10.1016/j.nicl.2019.101723. & Alzheimer’s Disease Neuroimaging Initiative
Zhang, H., Li, H., Yin, L., Chen, Z., Wu, B., Huang, X., Jia, Z., & Gong, Q. (2022). Aberrant White Matter Microstructure in Depressed patients with suicidality. Journal of Magnetic Resonance Imaging: JMRI, 55(4), 1141–1150. https://doi.org/10.1002/jmri.27927.
Zhou, M., Hu, Y., Huang, R., Zhou, Y., **e, X., Zhang, S., Jia, S., Zhang, Y., Xue, T., Dong, F., Lu, X., Yuan, K., & Yu, D. (2022). Right arcuate fasciculus and left uncinate fasciculus abnormalities in young smoker. Addiction Biology, 27(2), e13132. https://doi.org/10.1111/adb.13132.
Acknowledgements
Not applicable.
Funding
The study was supported by the General Projects of Jiangsu Commission of Health (No. H2018093). The Fifth Phase of “333 Project” Funded Scientific Research Project of Jiangsu Province (No. BRA2017175).
Author information
Authors and Affiliations
Contributions
Peng ZHANG, Yanhong FENG, Tianye XU, and Yifan LI contributed to the experiments, data collection, data analysis and writing of the manuscript. Zhongru Sun, Weizhong TIAN, Ji Zhang contributed to the data collection, performed the statistical analysis. Jianguo XIA, Hongxia ZHANG contributed to conception and study design, revising it critically for important intellectual content. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Affiliated Taizhou People’s Hospital of Nan**g Medical University. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, P., Feng, Y., Xu, T. et al. Brain white matter microstructural alterations in patients with systemic lupus erythematosus: an automated fiber quantification study. Brain Imaging and Behavior 18, 622–629 (2024). https://doi.org/10.1007/s11682-024-00861-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-024-00861-2