Abstract
Phase relations of Al-Ni-Zr ternary system were investigated with more than 60 ternary alloy compositions combining with x-ray diffraction, scanning electron microscopy equipped with energy dispersive spectroscopy and electron probe microanalysis. The isothermal section at 850 °C for x(Al) < 75 at.% has been constructed. The existence of five 2-phase regions and twenty-six 3-phase regions has been confirmed. In contrast to early reported in literature, two 3-phase equilibria: γ-(Ni) + Ni5Zr + γ′-AlNi3 and Ni5Zr + Ni7Zr2 + γ′ were determined. And a large solubility of Al in Ni7Zr2 about 11.07 at.% was detected. Besides, the 1050 °C isothermal section was determined at the Ni-rich corner, where an extended homogeneity of Ni7Zr2 was observed. Differences of the equilibria in the Ni-rich corner obtained in the present work and appeared in literatures were further analyzed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and Literature Review
Ni-based superalloys are being used in diverse range of applications for their excellent high temperature mechanical properties and corrosion resistance, e g. high temperature gas turbine hardware and corrosion resistant materials. Ni-Al binary is one of the most important systems for Ni-based superalloys as their characteristic microstructures consistent by disordered γ-(Ni) and order γ′-Ni3Al. As an important alloying element, addition of Zr can refine grain size and improve high temperature properties especially high temperature toughness for Ni3Al-based alloys.[1] On the other hand, Zr-based alloys are widely used as fuel cladding materials in light water reactors (LWRs) because of their low neutron absorption cross-section and high corrosion resistance.[2] In Zr-based alloys, addition of Al can enhance oxidation resistance owing to formation of dense alumina (Al2O3) on surface.[3] Similar to the effect of Fe and Cr, addition of Ni can promote mechanical properties of Zr-based alloys.[4] In addition, Al-Ni-Zr base system has attracted much interest in amorphous alloys due to their high glass-formation ability (GFA).[5,6]
In order to better understand alloy properties and develop new materials, knowledge concerning phase equilibria of Al-Ni-Zr alloy system is of fundamental importance. The constituent binary subsystems Al-Ni, Al-Zr and Ni-Zr have been extensively investigated both experimentally and thermodynamically. It was Nash and Jayanth[7] who first proposed a complete phase diagram for Ni-Zr system. Followed by Saunders,[8] Ghosh,[9] Yu,[10] Zaitsev[11] and Abe[12] have optimized the system utilizing different thermodynamic models. Recently, Wang[13] re-determined the eutectic temperature between NiZr and NiZr2 and re-assessed the system. The latest version of the Ni-Zr system contains 8 compounds including Ni5Zr, Ni7Zr2, Ni3Zr, Ni21Zr8, Ni10Zr7, Ni11Zr9, NiZr and NiZr2. For Al-Zr system, phase equilibria and crystal structure data prior to 1993 were reviewed and summarized by Okamoto[14] with new results for the phase relationships in Zr-rich part of Peruzzi.[15] Wang[16] assessed the system and reported ten stable linear compounds including Al3Zr, Al2Zr, Al3Zr2, AlZr, Al3Zr4, Al4Zr5, Al2Zr3, Al3Zr5, AlZr2 and AlZr3. This version of phase diagram was accepted by Guo[17] and Rigaud.[18] The Al-Ni binary phase diagram has been evaluated by Okamoto[19] in 1993, and successively optimized by Du,[20] Ansara,[21] Huang,[22] Dupin,[23] Lu[24] and Chen[25] respectively. Among those, the work of Dupin[23] appears to be the best and has been cited later by many authors,[26,27] of which five stable intermetallic phases being determined, i.e. AlNi3(γ), Al3Ni5, AlNi(β), Al3Ni2 and Al3Ni.
Phase relations in the Al-Ni-Zr system have been studied by many authors. It is accepted that seven ternary compounds: AlNiZr(τ1), AlNi2Zr(τ2), AlNi4Zr5(τ3), Al2NiZr6(τ4), Al15Ni8Zr6(τ5), Al5Ni2Zr(τ6) and Al2−x Ni x Zr(λ2) in this system(see Table 1). Mar[28] measured the isothermal section of Al-rich corner at 800 °C in 1966 and Bur[29] investigated the Zr-rich corner using microstructural and x-ray diffraction (XRD) techniques at the same temperature later. Phase equilibria at Ni-rich corner were determined by Jay[30,31] using metallography, electron microprobe analysis and x-ray powder diffraction techniques at 1000 and 1100 °C in 1983. Abovementioned results were reviewed by Nash[32] in 1991. Meanwhile, Nash[32] pointed out the contradiction of phase relations in Bur[29] and Jay[30,31] at Ni-rich corner and proposed a ternary eutectic reaction L ↔ γ + Ni5Zr + Ni7Zr2. However, Ghosh[33] pointed out that such a reaction is conflicting with the coexistence of γ′ and Ni5Zr observed by Miu[34] and proposed partial liquidus projection base on the result of Miu[34] in 2005. Later, Rag[35] reviewed briefly the liquidus projection at Ni-rich corner and also agreed with the result of Miu[34] in 2006. But, Mot[36] reported again a similar reaction (L ↔ γ + Ni5Zr + Ni7Zr2) to Nash[32] in 2011. In addition, as a stable phase at 800 °C, AlZr3 was not reported by Bur.[29]
Due to abovementioned issues associated with Al-Ni-Zr system, isothermal sections at 850 °C and at 1050 °C were studied and the liquidus projection in Ni-rich region was re-investigate with hope to clear the uncertainties and lay an experiment foundation to establish a self-consistent thermodynamic database.
Prior to submission, we are delighted to find out the work of Kondrat’ev et al. on (partial)Phase Equilibria in Al-Ni-Zr System at 1123 K (850 °C)[37] which is consistence with one of the selected temperatures of us and we will point out the difference in section 3.
Experimental
More than 60 ternary alloy compositions were studied to measure phase relations in this study. All samples were fabricated from starting materials: aluminum grain (99.99%), nick rod (99.95%) and zirconium pieces (99.99%). Weight of each sample is limited to about 5 gram. Predetermined amount of each raw material was weighed by analytical balance, followed by arc-melted on a water-cooled copper plate under argon atmosphere with titanium as getter material placed in the arc chamber. Each sample was melted three or four times for homogenization and re-weighed to check for possible mass loss (in our case, mass lose is negligible). Subsequently, the samples were sealed in evacuated quartz tubes and then heat-treated according to different annealing processes.
60 samples covering entire composition range were annealed at 850 °C for 50 days and 19 samples used to determine the phase equilibria at Ni-rich corner were annealed at 1050 °C for 30 days respectively. After annealing, all samples were quenched in cold water.
The annealed specimens were polished and investigated by x-ray powder diffraction and scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS) for initial phase relationship analysis. Some typical annealed samples and a set of as-cast samples were further analyzed by electron probe microanalysis (EPMA) for precise determination of chemical compositions of multiple coexisting phases.
Results and Discussions
Isothermal Section at 850 °C
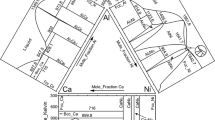
The isothermal section at 850 °C was studied covering almost the entire composition range in order to establish the thermodynamic database of Al-Ni-Zr system later. The typical measured compositions of phases in equilibrium are listed in Table 2. For more precise determination of solubility for phase boundary of γ′, Ni7Zr2, λ2 and τ5, EPMA measurements were carried out on selected samples. Based on the experimental results, the isothermal section of Al-Ni-Zr ternary system at 850 °C has been constructed as shown in Fig. 1.
Back-scattered electron (BSE) images of typical ternary Al-Ni-Zr samples annealed at 850 °C are presented in Fig. 2. At Zr-rich corner, in sample #13 (Al-41Ni-47Zr), three phase microstructure is observed, as showed in Fig. 2(a). Preliminary EDS analysis indicate that the dark phase is τ1, the gray one is ternary τ3, while the bright one is NiZr. This result is confirmed by XRD (see Fig. 3(a)). It is worth mentioning here that τ3 was not reported until 2006 by Leineweber[38] and JADE 5 does not contain corresponding PDF card. In order to determine whether it is τ3 or not, PDF Card of τ3 was created according to its structure data pointed by FindIt2010, PowderCell and JADE 5. Similar method was used for determining τ3 in samples #14, #15 and #16. Figure 2(b) shows three phase microstructure of sample #15 (Al-27Ni-52Zr). Initial EDS analysis showed that the dark phase is τ1, the gray one is τ3 and the light gray one is τ4. These results have been confirmed by XRD analysis (see Fig. 3(b)).
Note here, many phase relationships determined in present work are basically in consistence with those reported by Bur[29] at 800 °C with one exception at Zr-rich corner. A stable AlZr3 at 850 °C was detected in samples #20 and #21 (see Table 2), which was not occurred in the section of Bur.[29] According to phase diagram of Al-Zr binary system, AlZr3 is a stable phase from room temperature up to 1019 °C, which is formed from eutectoid reaction AlZr2 + Zr(bcc) ↔ AlZr3.
BSE image of sample #3 (Al-77.5Ni-19Zr) locating at Ni-rich corner is presented in Fig. 2(c) showing coexistence of three phases, of which the black one is AlNi3(γ′), the gray one is Ni5Zr and the greyish-white one is Ni7Zr2. This is confirmed with XRD pattern as illustrated in Fig. 3(c). The phase equilibrium, i.e. γ′ + Ni5Zr + Ni7Zr2, differs from that obtained in Bur[29] and Jay.[30,31] As pointed out by Nash,[32] the number of samples measured by Bur[29] is not enough to obtain exact phase relation. So, we thought that the phase equilibrium should be Ni5Zr + Ni7Zr2 + γ′. Figure 2(d) and 3(d) show BSE image and XRD pattern of sample #9 (Al-52Ni-11Zr). By SEM-EDS analysis it is known that there is a three-phase region consisted of bright Ni7Zr2, gray τ2 and dark AlNi(β), further confirmed through XRD analysis. This three-phase region also existed in the isothermal sections of Jay,[30,31] but did not appear in that of Bur.[29] In light of the review of Nash,[32] the presently measured equilibrium Ni7Zr2 + τ2 + AlNi(β) should be accepted. In addition, the solubility of Al in Ni7Zr2 is further analyzed (sample #9) is up to 11.07 at.% at 850 °C.
In the x(Al) > 50 at.% region, phase relations are similar to those established by Bur,[29] but τ5 phase exhibits similar shape of homogeneity regions as that of λ2. In the three-phase equilibrium of (τ1 + τ5 + β), the composition (at.%) of τ5 is 51.5Al-28.7Ni-19.9Zr while in the (τ5 + τ6 + λ2) equilibrium, τ5 has the composition of 57.3Al-23.9Ni-18.8Zr. Similarly, λ2 shows the composition of 64.5Al-5.2Ni-30.3Zr in the (λ2 + Al2Zr + Al3Zr) equilibrium and 50.7Al-17.7Ni-31.6Zr in the (λ2 + τ1 + Al2Zr3) equilibrium. So far, twenty-six 3-phase regions and five 2-phase regions have been determined in Al-Ni-Zr system at 850 °C section (see Table 2).
According to Ref 37, except minor difference in solubility of Al in Ni7Zr2, their partial section of 850 °C (1123 K) (in their Fig. 2) at Zr-Ni side (0-45 at.% Al) can be fitted nicely into our Fig. 1. In another word, those ambiguities and arguments mentioned previously can be clarified with our current work.
Isothermal Section at 1050 °C
Only phase relations in Ni-rich corner at 1050 °C were determined due to possible formation of liquid at both Al-rich and Zr-rich corners. The selected compositions of equilibrium phases coexisting in different samples are listed in Table 3. The isothermal section of the Al-Ni-Zr system at 1050 °C is constructed and presented in Fig. 4.
Microstructures of some typical samples annealed at 1050 °C for 30 days were demonstrated in Fig. 5. BES image of sample #1 (Al-83.6Ni-5Zr) is presented in Fig. 5(a) with two distinct phases observable. According to EDS analysis, the dark phase is γ(Ni) while the gray one is Ni5Zr phase. XRD pattern of this sample in Fig. 6(a) indicates coexistence of Ni5Zr and γ phases. Note here, the determined phase region of Ni5Zr + γ is wider than that reported previously.[30,31]
The BES micrograph of sample #2 (Al-61.6Ni-7Zr) is showed in Fig. 5(b), where two different phases (dark and bright phases) are distinguished. Initial EDS analysis suggested that the dark phase is β and the bright one is Ni7Zr2, agreeing with XRD analysis as showed in Fig. 6(b). This phase region (β + Ni7Zr2) observed here is consistent with the phase relationships determined by Jay.[30,31]
In sample #5 (Al-80Ni-10Zr) quenched from 1050 °C, a three phase microstructure was observed (Fig. 5(c)). EDX result indicated the white phase is Ni5Zr, the darkish one is γ(Ni) and the black one is γ′(AlNi3). The XRD pattern of this sample is presented in Fig. 6(c), confirming coexistence of these three phases. Note here, the contrast of γ and γ′ shows little difference in Fig. 5(c) due to the small difference between their compositions. The phase equilibrium (γ + γ′ + Ni5Zr) observed here is different from the phase relation (γ + γ′ + Ni7Zr2) reported by Jay.[30,31] Ghosh[33] pointed out that the equilibria obtained by Jay[30,31] were not exact due to inconsistency between their x-ray data and electron microprobe analysis. In addition, this phase equilibrium (γ + γ′ + Ni5Zr) has also been observed in our work at 850 °C. Thus, such a phase equilibrium (γ + γ′ + Ni5Zr) determined here is reasonable.
Figure 5(d) present BES image of sample #6 (Al-65Ni-2Zr), of which three different phases, i.e. γ′, β and Ni7Zr2, were identified by EDS analysis. XRD pattern confirms coexistence of these three phases in this sample as illustrated in Fig. 6(d). Combining #6 and #7 samples, it is found that Ni7Zr2 phase has a similar solubility (12.24%) as we determined at 850 °C. In addition, from the results of samples #2 and #3, it is found that certain amount of Zr can be solved in γ′ but not in β where the solubility is negligible.
In summary, the 1050 °C isothermal section of Al-Ni-Zr system has been determined, where six 3-phase regions and three 2-phase regions being confirmed (see Table 3) as demonstrated in Fig. 4.
Projection in the Ni-Rich Portion
Until now, phase relations of projection in Ni-rich region have been investigated by many researchers. But there are still arguments remained unclear as mentioned before. A set of as-cast samples were further investigated to clarify phase relationship for Ni-rich portion of this system. The compositions of the samples tested are listed in Table 4.
BES micrograph and XRD-pattern of as-cast sample #1 (Al-75Ni-12.5Zr) are showed in Fig. 7(a) and (b), respectively. EPMA analysis suggested that the black phase is γ′, the gray one is γ and the white one is Ni5Zr. According to the microstructure in Fig. 7(a), a solidification path can be inferred, with large primary phase γ′ solidified from the liquid first, L → γ′; after that the liquid composition reached the univariant line, L → γ′ + Ni5Zr; finally, the liquid composition reached the eutectic invariant reaction, L → γ′ + Ni5Zr + γ.
Figure 7(c) is the BES image of sample #2 (Al-77.5Ni-19Zr). The result of EPMA indicates that white-gray phase is Ni7Zr2, the gray one is Ni5Zr and the black one is γ′. Similar to sample #1, an equilibrium solidification path is proposed. During solidification, the white-gray primary phase (Ni7Zr2) first solidified through L → Ni7Zr2, followed by the binary peritectic reaction L + Ni7Zr2 → Ni5Zr when liquid composition reached the peritectic univariant line. Finally, Ni5Zr + γ′ forms through peri-eutectic reaction L + Ni7Zr2 → Ni5Zr + γ′. However, due to slow diffusion of atoms across solid compound, e.g. Ni7Zr2, peritectic-type reaction may not reach completion, and Ni5Zr may crystallize directly from remained liquid. Finally, the eutectic reaction L → Ni5Zr + γ′ takes place when liquid composition reach the univariant curve.
Composition of sample #3 (Al-77Ni-13Zr) is identical to that in Miu.[34] According to EPMA results, the white primary phase is Ni7Zr2, the eutectic microstructure is consistent of gray one (Ni5Zr) and black one (γ′), as shown in Fig. 7(d). It is thought that Ni7Zr2 forms primarily from liquid, then the residual liquid solidifies through the reactions L + Ni7Zr2 → Ni5Zr + γ′ and L → Ni5Zr + γ′. As the sample composition approaching to the invariant reaction point, a tiny primary Ni7Zr2 was formed, with microstructure consisting mainly of γ′ + Ni5Zr as those observed in Miu.[34]
In brief, according to as-cast microstructure analysis of additional samples above, there may exist two invariant reactions, i.e. L + Ni7Zr2 → Ni5Zr + γ′ and L → γ′ + Ni5Zr + γ, in the projection of Ni-rich region of the Al-Ni-Zr system.
The liquid projections in the Ni-rich portion from literatures are shown in Fig. 8. Ghosh[33] disagree with the liquidus projection proposed by Nash[32] based on the microstructure (γ′ + Ni5Zr) observed by Miu.[34] But this microstructure (γ′ + Ni5Zr) may not as a proof to oppose Nash[32] because the sample used by Miu[34] being annealed at 1000 °C for 6 days. Later, Mot[36] proposed the similar liquidus projection as given by Nash[32] after analyzed the microstructure he observed and compared with the projection of Al-Ni-Hf system. Nevertheless, the alloys examined by Mot[36] contained other elements such as Cr, Mo and B, implying that as-cast microstructure observed by Mot[36] may not qualify as direct proof to explain the solidification path of the Al-Ni-Zr alloys.
Combining with the results of this work, the projection given by Nash[32] may not be able to explain the eutectic microstructure (Ni5Zr + γ′) observed here. But, the projections deduced by Ghosh[33] and Rag[35] seem to be reasonable, and can explain the solidification path of our as-cast samples. So, the projections proposed by Ghosh[33] and Rag[35] are accepted here.
Conclusions
The isothermal section of Al-Ni-Zr ternary system at 850 °C and partial isothermal section at 1050 °C have been determined. A reasonable phase relationship has been determined at the Ni-rich corner of the sections in Al-Ni-Zr ternary system. The solubility of Al in Ni7Zr2 can be up to about 11.07% at 850 °C and approximately 12.24% at 1050 °C. An extended homogeneity of τ5 is observed at 850 °C. AlZr3 phase is stable in isothermal section of Al-Ni-Zr ternary system at 850 °C.
References
L.P. Zheng, D.X. Li, and X.R. Zhang, Simulations of Bulk Effects of Zr on Cohesion of Ni3Al Grain Boundary, Nucl. Phys. Rev., 2005, 22, p 106-109
S. Yamada and T. Kameyama, Observation of c-Component Dislocation Structures Formed in Pure Zr and Zr-Base Alloy by Self-Ion Accelerator Irradiation, J. Nucl. Mater., 2012, 442, p 167-172
T. Cheng, J.R. Keiser, and P. Michael, Oxidation of Fuel Cladding Candidate Materials in Steam Environments at High Temperature and Pressure, J. Nucl. Mater., 2012, 427, p 396-400
S.L. Wadekar, V.V. Raman, S. Banjerjee, and M.K. Asundi, Structure-Property Correlation of Zr-Base Alloys, J. Nucl. Mater., 1988, 151, p 162-171
H.W. Choi, J.H. Cho, J.E. Kim, Y.H. Kim, and Y.S. Yang, Calorimetric and Structural Properties of Amorphous Zr-Al-Ni Alloys, Scr. Mater., 2001, 44, p 2027-2030
Y. Wang, C.H. Shek, and J. Qiang, The e/a Criterion for the Largest Glass-forming Abilities of the Zr-Al-Ni(Co) Alloys, Mater. Trans., 2004, 45(4), p 1180-1183
C.S. Jayanth and P. Nash, The Ni-Zr System, J. Phase Equilib., 1984, 5(2), p 144-148
N. Saunders, Phase Diagram Calculations for Eight Glass Forming Alloy Systems, CALPHAD, 1985, 9, p 297-309
G. Ghosh, Thermodynamics and Kinetics of Stable and Metastable Phases in the Ni-Zr System, J. Mater. Res., 1994, 9, p 598-616
H. Yu and Z.P. **, Thermodynamic Assessment of the Ni-Zr Equilibrium Phase Diagram, Chin. J. Nonferrous Met., 1997, 7, p 107-111
A.I. Zaitsev, N.E. Zaitseva, and E.Kh. Shakhpazov, Thermodynamic Properties and Phase Equilibria in the Nickel-Zirconium System. The Liquid to Amorphous State Transition, Phys. Chem. Chem. Phys., 2002, 4, p 6047-6058
T. Abe, H. Onodera, M. Shimono, and M. Ode, Thermodynamic Modeling of the Undercooled Liquid in the Ni-Zr System, Mater. Trans., 2005, 46, p 2838-2843
N. Wang, C. Li, Z. Du, and F. Wang, Experimental Study and Thermodynamic Re-Assessment of the Ni-Zr System, CALPHAD, 2007, 31, p 413-421
H. Okamoto, Al-Zr (Aluminum-Zirconium), J. Phase Equilib., 1993, 14, p 259-260
A. Peruzzi, Reinvestigation of the Zr-Rich End of the Zr-Al Equilibrium Phase Diagram, J. Nucl. Mater., 1992, 186, p 89-99
T. Wang, Z.P. **, and J.C. Zhao, Thermodynamic Assessment of the Al-Zr Binary System, J. Phase Equilib., 2001, 22, p 544-551
Z.M. Du, C.P. Guo, C.R. Li et al., Thermodynamic Description of Al-Fe-Zr System, CALPHAD, 2008, 32, p 637-649
B. Sundman, V. Rigaud, D. Daloz et al., Thermodynamic Assessment of the Fe-Al-Zr Phase Diagram, CALPHAD, 2009, 33, p 442-449
H. Okamoto, Al-Ni (Aluminum-Nickel), J. Phase Equilib., 1993, 14, p 257-259
Y. Du and N. Clavaguera, Thermodynamic Assessment of the Al-Ni System, J. Alloys Compd., 1996, 6, p 487-498
N. Dupin, I. Ansara, H.L. Lukas et al., Thermodynamic Assessment of Al-Ni System, J. Alloys Compd., 1997, 247, p 20-30
Y.A. Chang and W. Huang, A Thermodynamic Analysis of the Ni-Al System, Intermetallics, 1998, 6, p 187-498
N. Dupin, I. Ansara, and B. Sundman, Thermodynamic Re-Assessment of the Ternary System Al-Cr-Ni, CALPHAD, 2001, 25, p 279-298
B. Sundman, X.G. Lu, and J. Agren, Thermodynamic Assessments of the Ni-Pt and Al-Ni-Pt Systems, CALPHAD, 2009, 33, p 450-456
H.-L. Chen, E. Doernberg, P. Svoboda, and R. Schmid-Fetzer, Thermodynamics of the Al3Ni Phase and Revision of the Al-Ni System, Thermochim. Acta, 2011, 512, p 189-195
L.J. Zhang, J. Wang, Y. Du, R.X. Hu, P. Nash et al., Thermodynamic Properties of the Al-Fe-Ni System Acquired Via a Hybrid Approach Combining Calorimetry, First-Principles and CALPHAD, Acta Mater., 2009, 57, p 5324-5341
Y. Du, Y.A. Chang, W.P. Gong et al., Thermodynamic Properties of the Al-Nb-Ni System, Intermetallics, 2003, 11, p 995-1013
V.Ya. Markiv, N.E. Matushevskaya, S.N. Rozum, and Yu.B. Kuzma, Investigation of Al-Rich Alloys of the System Zr-Ni-Al, Akad. Nauk SSSR, Neorg. Mater., 1966, 2, p 1356-1359
V.V. Burnashova and V.Ya. Markiv, Zirconium-Nickel-Aluminum System, Visn. L’viv. Derzh. Univ. Ser. Khint., 1969, 11, p 34-37
C.S. Jayanth, Phase Equilibria in the Ni-AI-Zr and Ni-Al-V Systems, M.S. Thesis, Illinois Institute of Technology, 1983.
C.S. Jayanth and P. Nash, Phase Equilibria in the Ni-Rich Region of the Ni-Al-Zr System, Mater. Res. Soc. Symp. Proc., 1983, 19, p 395-398
P. Nash and Y.Y. Pan, The Al-Ni-Zr (Aluminum-Nickel-Zirconium) System, J. Phase Equilib., 1991, 12, p 105-113
G. Ghosh, in Aluminum-Nickel-Zirconium, Light Metal Ternary Systems, 11A3, G. Effenberg and S. Ilyenko, Eds., Springer, Heidelberg, 2005, p 451-463
S. Miura, H. Unno, T. Yamazaki, S. Takizawa, and T. Mohri, Reinvestigation of Ni-Solid Solution/Liquid Equilibria in Ni-Al Binary and Ni-Al-Zr Ternary Systems, J. Phase Equilib., 2001, 22(4), p 457-462
V. Raghavan, Al-Ni-Zr (Aluminum-Nickel-Zirconium), J. Phase Equilib. Diffus., 2006, 27(5), p 507
H.B. Motejadded, M. Soltanieh, and S. Rastegari, Dissolution Mechanism of a Zr Rich Structure in a Ni3Al Base Alloy, J. Mater. Sci. Technol., 2011, 27, p 885-892
D.M. Kondrat’ev, K.B. Kalmykov, N.E. Dmitrieva, and S.F. Dunaev, Phase Equilibria in Al-Ni-Zr System at 1123 K, Mosc. Univ. Chem. Bull., 2012, 67, p 259-264
H. Nitsche, A. Leineweber, V. Hlukhyy et al., Zirconium-Aluminium Ordering in Zr5Ni4Al—A New Superstructure in the Family of U3Si2 Related Intermetallics, Intermetallics, 2006, 14, p 685-694
A.E. Dwight, M.H. Mueller, R.A. Conner, J.W. Downey, and H. Knott, Ternary Compounds with the Fe2P-Type Structure, Trans. TMS AIME, 1968, 242, p 2075-2080
F.S. Da Rocha, G.L.F. Fraga et al., Specific Heat and Electronic Structure of Heusler Compounds Ni2TAl (T = Ti, Zr, Hf, V, Nb, Ta), Physica B, 1999, 269, p 154-162
V.Ya. Markiv, P.I. Kripyakevich, and N.M. Belyavina, Crystal Structure of the Compound ZrNi2Al5 (in Ukrainian), Dopov. Akad. Nauk Ukr. RSR, 1982, 3, p 76-78
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant No. 51171210).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Huang, L.H., Liu, H.S. et al. Isothermal Sections of Al-Ni-Zr Ternary System at 850 and 1050 °C. J. Phase Equilib. Diffus. 34, 390–402 (2013). https://doi.org/10.1007/s11669-013-0248-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-013-0248-8