Abstract
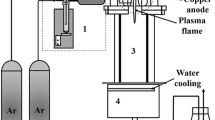
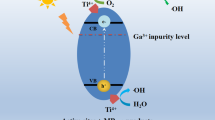
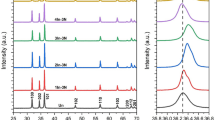
N-doped ZnO/zinc titanate core–shell nanorod arrays (CSNAs) were prepared via simple aqueous solution methods and microwave nitrogen plasma treatment. The impacts of nitrogen do** on the phase structure, band gap and photocatalytic efficiency of the CSNAs were also investigated. After nitrogen plasma treatment, the phase transition of zinc titanate shell layer from cubic Zn2TiO4 to hexagonal ZnTiO3 was demonstrated. X-ray photoelectron spectroscopy indicates that the atomic percentage of the interstitial N is almost unchanged with the increase of the plasma treatment time, while the atomic percentage of the substitutional N increases significantly. It has been found that the optical band gap of N-doped CSNAs is obviously decreased with the increasing of the concentration of the substitutional N. The photocatalytic efficiency of the N-doped CSNAs increases with the concentration of substitutional N under visible-light irradiation. However, when the concentration of substitutional N exceeds 2.34%, the photocatalytic efficiency decreases. The high visible-light photocatalytic efficiency is attributed to the enhancement of visible-light absorption and effective separation of the photogenerated carriers. And the excessive oxygen vacancies introduced by higher concentration of substitutional N lead to the decrease of the photocatalytic activity.







Similar content being viewed by others
References
P. Longchin, S. Sakulsermsuk, K. Wetchakun, and N. Wetchakun, Synergistic effect of La and Mo co-do** on the enhanced photocatalytic activity of Bi2WO6. Mater. Lett. 305, 130779 (2021).
Z. Zhang, C.S. Feng, C.Y. Jiang, and Y.P. Wang, Enhanced visible-light photocatalytic activity and mechanism of Ag@AgCl-decorated TiO2 nanotubes. J. Electron. Mater. 50, 352 (2021).
Y.F. Tu, Q.M. Fu, X.J. Niu, J.P. Sang, Z.J. Tan, and X.W. Zou, Facile synthesis of SnO2 nanotube arrays by using ZnO nanorod arrays as sacrificial templates. J. Mater. Sci. Technol. 29, 1053 (2013).
J.X. Lu, D.L. Li, Y. Chai, L. Li, M. Li, Y.Y. Zhang, and J. Liang, Rational design and preparation of nanoheterostructures based on zinc titanate for solar-driven photocatalytic conversion of CO2 to valuable fuels. Appl. Catal., B 256, 117800 (2019).
R. Abirami, T.S. Senthil, S.P. Keerthana, R. Yuvakkumar, G. Ravi, M. Pannipara, and A.G. Al-Sehemi, An approach to enhance the photocatalytic activity of ZnTiO3. Ceram. Int. 47, 18122 (2021).
D.C. He, Q.M. Fu, Z.B. Ma, H.Y. Zhao, Y.F. Tu, Y. Tian, D. Zhou, G. Zheng, and H.B. Lu, Facile synthesis and photocatalytic activity of ZnO/zinc titanate core-shell nanorod arrays. Mater. Res. Express 5, 025006 (2018).
Y.Y. Cai, Y.X. Ye, Z.F. Tian, J. Liu, Y.S. Liu, and C.H. Liang, In situ growth of lamellar ZnTiO3 nanosheets on TiO2 tubular array with enhanced photocatalytic activity. Phys. Chem. Chem. Phys. 15, 20203 (2013).
H.S. Lim, J. Lee, S. Lee, Y.S. Kang, Y.K. Sun, and K.D. Suh, Walnut-like ZnO@Zn2TiO4 multicore-shell submicron spheres with a thin carbon layer: fine synthesis, facile structural control and solar light photocatalytic application. Acta Mater. 122, 287 (2017).
H.F. Zhuang, C.J. Lin, Y.K. Lai, L. Sun, and J. Li, Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environ. Sci. Technol. 41, 4735 (2007).
F.A. Hernández García, G. Torres Delgado, R. Castanedo Pérez, and O. Zelaya Ángel, Gaseous benzene degradation by photocatalysis using ZnO+Zn2TiO4 thin films obtained by sol-gel process. Environ. Sci. Pollut. Res. 23, 13191 (2016).
J.S. Jang, P.H. Borse, J.S. Lee, K.T. Lim, O.S. Jung, E.D. Jeong, J.S. Bae, M.S. Won, and H.G. Kim, Energy band structure and photocatalytic property of Fe-doped Zn2TiO4 material. Bull. Korean Chem. Soc. 30, 3021 (2009).
X. Sun, S. Wang, C. Shen, and X. Xu, Efficient photocatalytic hydrogen production over Rh-doped inverse spinel Zn2TiO4. ChemCatChem 8, 2289 (2016).
S. Singh, S. Perween, and A. Ranjan, Enhancing the visible light induced photocatalytic activity in sol-electrospun zinc titanate nanopowders by nitrogen do**. J. Phys. Chem. Solids 158, 110221 (2021).
J.C. Conesa, Band structures and nitrogen do** effects in zinc titanate photocatalysts. Catal. Today 208, 11 (2013).
Y.F. Tu, Q.M. Fu, X.J. Niu, J.P. Sang, Z.J. Tan, G. Zheng, and X.W. Zou, Fabrication and photocatalytic property of ZnO/SrTiO3 core/shell nanorod arrays. Cryst. Res. Technol. 48, 138 (2013).
J.H. Lee, I.C. Leu, M.C. Hsu, Y.W. Chung, and M.H. Hon, Fabrication of aligned TiO2 one-dimensional nanostructured arrays using a one-step templating solution approach. J. Phys. Chem. B 109, 13056 (2005).
S. Yodyingyong, X.Y. Zhou, Q.F. Zhang, D. Triampo, J.T. **, K. Park, B. Limketkai, and G.Z. Cao, Enhanced photovoltaic performance of nanostructured hybrid solar cell using highly oriented TiO2 nanotubes. J. Phys. Chem. C 114, 21851 (2010).
B. Lokesh, and N.M. Rao, Effect of Cu-do** on structural, optical and photoluminescence properties of zinc titanates synthesized by solid state reaction. J. Mater. Sci.: Mater. Electron. 27, 4253 (2016).
Q. Li, and J.K. Shang, Composite photocatalyst of nitrogen and fluorine codoped titanium oxide nanotube arrays with dispersed palladium oxide nanoparticles for enhanced visible light photocatalytic performance. Environ. Sci. Technol. 44, 3493 (2010).
D.L. Shieh, Y.S. Lin, J.H. Yeh, S.C. Chen, B.C. Lin, and J.L. Lin, N-doped, porous TiO2 with rutile phase and visible light sensitive photocatalytic activity. Chem. Commun. 48, 2528 (2012).
J. Yu, D. Li, L.X. Zhu, and X.L. Xu, Application of ZnTiO3 in quantum-dot-sensitized solar cells and numerical simulations using first-principles theory. J. Alloys Compd. 681, 88 (2016).
C. Wattanawikkam, and W. Pecharapa, Sonochemical synthesis, characterization, and photocatalytic activity of perovskite ZnTiO3 nanopowders. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 63, 1663 (2016).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 11404248, No. 11304124), and the Graduate Innovative Fund of Wuhan Institute of Technology (No. CX2020158).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, QM., Dong, HK., Li, WL. et al. Effects of N Do** on Phase Transition and Visible-Light Photocatalytic Activity of ZnO/Zinc Titanate Core–Shell Nanorod Arrays. J. Electron. Mater. 51, 2599–2607 (2022). https://doi.org/10.1007/s11664-022-09532-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09532-8