Abstract
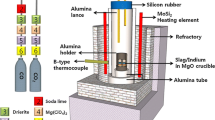
To establish the dissolution mechanism of palladium (Pd) in the FetO–SiO2–CaO–Al2O3–MgOsat slag system, the Pd solubility was measured at 1873 K (1600 °C) and oxygen partial pressure ranging from \({{p}_{\text{O}_{2}}} = 1.0\times {10}^{-8}\,{\text{atm}}\) to \({{p}_{\text{O}_{2}}} = 1.0\times {10}^{-6}\,{\text{atm}}\). In the current slag system, the Pd solubility increases with increasing content of CaO and FeO, and decreases with increasing content of SiO2 and Al2O3. This solubility behavior is quantified by employing the modified Vee ratio, defined as (CaO+FeO)/(SiO2+Al2O3), the theoretical optical basicity and the activity of CaO in the slag. Thermodynamic analysis reveals that Pd stabilizes in the form of the (\({{\text{PdO}}}_{2}^{3-}\)) palladate complex ion in the present aluminosilicate melts. Based on this, the Pd dissolution reaction in the current slag system is proposed as follows:
\({\text{Pd}}(l)+\frac{1}{4}{{\text{O}}}_{2}(g)+\frac{3}{2}\left({{\text{O}}}^{2-}\right)=({{\text{PdO}}}_{2}^{3-})\)
Combining the results from the present experiment and previous findings suggests that a well-designed slag system can effectively minimize Pd loss during the pyrometallurgical processing of industrial wastes containing Pd. By adjusting the modified Vee ratio, in which CaO and FeO promote Pd solubility in the slag, whereas SiO2 increases slag viscosity, it is possible to achieve optimal conditions. Therefore, we recommend implementing such an optimized slag system for pyrometallurgical treatment of Pd-containing industrial wastes, ensuring minimal Pd loss and maximizing resource recovery.











Similar content being viewed by others
References
R. Widmer, H. Oswald-Krapf, D. Sinha-Khetriwal, M. Schnellmann, and H. Böni: Environ. Impact Assess. Rev., 2005, vol. 25, pp. 436–58. https://doi.org/10.1016/j.eiar.2005.04.001.
V. Forti, C.P. Balde, R. Kuehr, and G. Bel: The Global E-waste Monitor 2020, United Nations University (UNU), 2020.
M. Kaya: Energy Technology 2017, The Minerals, Metals & Materials Society, 2017, pp. 433–51. https://doi.org/10.1007/978-3-319-52192-3_43
K. Liu, Q. Tan, J. Yu, and M. Wang: Circ. Econ., 2023, vol. 2, art no. 100028. https://doi.org/10.1016/j.cec.2023.100028.
Y. Zhou and K. Qiu: J. Hazard. Mater., 2010, vol. 175, pp. 823–28. https://doi.org/10.1016/j.jhazmat.2009.10.083.
J.H. Heo, J. Park, and J.H. Park: Resour. Conserv. Recycl., 2022, vol. 179, art no. 106068. https://doi.org/10.1016/j.resconrec.2021.106068.
S.J. Song, V.N.H. Nguyen, and M.S. Lee: Kor. J. Met. Mater., 2022, vol. 60, pp. 188–97. https://doi.org/10.3365/KJMM.2022.60.3.188.
S.J. Song, V.N.H. Nguyen, and M.S. Lee: Kor. J. Met. Mater., 2022, vol. 60, pp. 837–44. https://doi.org/10.3365/KJMM.2022.60.11.837.
S. Nakamura and N. Sano: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 103–08. https://doi.org/10.1007/s11663-997-0132-1.
H. Shuto, T.H. Okabe, and K. Morita: Mater. Trans., 2011, vol. 52, pp. 1899–904. https://doi.org/10.2320/matertrans.M-M2011821.
C. Wiraseranee, T.H. Okabe, and K. Morita: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 584–92. https://doi.org/10.1007/s11663-013-9816-x.
K. Morita, C. Wiraseranee, H. Shuto, S. Nakamura, K. Iwasawa, T.H. Okabe, and N. Sano: Miner. Process. Extr. Metall. (Trans. Inst. Min. Metall. C), 2014, vol. 123, pp. 29–34. https://doi.org/10.1179/0371955313Z.00000000070.
A. Borisov, H. Palme, and B. Spettel: Geochim. Cosmochim. Acta, 1994, vol. 58, pp. 705–16. https://doi.org/10.1016/0016-7037(94)90500-2.
N.A. Sullivan, Z. Zajacz, and J.M. Brenan: Geochim. Cosmochim. Acta, 2018, vol. 231, pp. 15–29. https://doi.org/10.1016/j.gca.2018.03.019.
V. Laurenz, R.O.C. Fonseca, C. Ballhaus, and P.J. Sylvester: Geochim. Cosmochim. Acta, 2010, vol. 74, pp. 2989–98. https://doi.org/10.1016/j.gca.2010.02.015.
K. Avarmaa, L. Klemettinen, H. O’Brien, A. Jokilaakso, D. Lindberg, and P. Taskinen: JOM, 2021, vol. 73, pp. 1871–77. https://doi.org/10.1007/s11837-021-04672-4.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, 1980, pp. 1–24.
K.Y. Ko and J.H. Park: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 1224–30. https://doi.org/10.1007/s11663-011-9566-6.
K.Y. Ko and J.H. Park: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 440–42. https://doi.org/10.1007/s11663-012-9649-z.
Y.S. Han and J.H. Park: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 235–42. https://doi.org/10.1007/s11663-014-0209-6.
Y.S. Han, D.R. Swinbourne, and J.H. Park: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 2449–57. https://doi.org/10.1007/s11663-015-0421-z.
J.G. Yang, J.H. Park, J.Y. Kang, H.S. Park, and J.H. Park: JOM, 2021, vol. 73, pp. 688–93. https://doi.org/10.1007/s11837-020-04527-4.
J.H. Park and G.H. Park: ISIJ Int., 2012, vol. 52, pp. 764–69. https://doi.org/10.2355/isi**ternational.52.764.
Y.B. Kang and J.H. Park: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 1211–17. https://doi.org/10.1007/s11663-011-9541-2.
J.H. Park, G.H. Park, and Y.E. Lee: ISIJ Int., 2010, vol. 50, pp. 1078–83. https://doi.org/10.2355/isi**ternational.50.1078.
M.K. Cho, J. Cheng, J.H. Park, and D.J. Min: ISIJ Int., 2010, vol. 50, pp. 215–21. https://doi.org/10.2355/isi**ternational.50.215.
J.H. Heo, S.S. Park, and J.H. Park: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 1098–105. https://doi.org/10.1007/s11663-012-9701-z.
J.A. Duffy and M.D. Ingram: J. Non-Cryst. Solids, 1976, vol. 21, pp. 373–410. https://doi.org/10.1016/0022-3093(76)90027-2.
J.A. Duffy and M.D. Ingram: J. Am. Chem. Soc., 1971, vol. 93, pp. 6448–54. https://doi.org/10.1021/ja00753a019.
J.A. Duffy, M.D. Ingram, and I.D. Sommerville: J. Chem. Soc. Faraday Trans., 1978, vol. 74, pp. 1410–19. https://doi.org/10.1039/F19787401410.
J.H. Park and D.J. Min: J. Non-Cryst. Solids, 2004, vol. 337, pp. 150–56. https://doi.org/10.1016/j.jnoncrysol.2004.03.109.
J.H. Park, H. Kim, and D.J. Min: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 150–53. https://doi.org/10.1007/s11663-007-9122-6.
J.H. Park, H. Kim, and D.J. Min: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 269–75. https://doi.org/10.1007/s11663-004-0028-2.
J.S. Choi, T.J. Park, and D.J. Min: J. Am. Ceram. Soc., 2021, vol. 104, pp. 140–56. https://doi.org/10.1111/jace.17432.
L. Zhang and S. Jahanshahi: Scand. J. Metall., 2001, vol. 30, pp. 364–69. https://doi.org/10.1034/j.1600-0692.2001.300603.x.
S. Sukenaga, N. Saito, K. Kawakami, and K. Nakashima: ISIJ Int., 2006, vol. 46, pp. 352–58. https://doi.org/10.2355/isi**ternational.46.352.
T.S. Kim and J.H. Park: J. Alloys Compd., 2022, vol. 916, art no. 165328. https://doi.org/10.1016/j.jallcom.2022.165328.
D.S. Goldman: J. Am. Ceram. Soc., 1983, vol. 66, pp. 205–09. https://doi.org/10.1111/j.1151-2916.1983.tb10018.x.
T. Førland and K. Grjotheim: Metall. Trans. B, 1978, vol. 9B, pp. 45–49. https://doi.org/10.1007/BF02822670.
www.factsage.com (February 2024).
C.J.B. Fincham and F.D. Richardson: Proc. R. Soc. Lond., 1954, vol. 223A, pp. 40–62.
H.S. Park, Y.S. Han, and J.H. Park: ACS Sustain. Chem. Eng., 2019, vol. 7, pp. 14119–25. https://doi.org/10.1021/acssuschemeng.9b02725.
Acknowledgments
This work was supported by the Competency Development Program for Industry Specialists from the Korea Institute for Advancement of Technology (KIAT, Grant No. P0023676) and the Korea Institute of Energy Technology Evaluation and Planning (KETEP, Grant No. 20217510100080), funded by the Ministry of Trade, Industry and Energy (MOTIE), Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, R.R., Kim, H.J., Park, H.S. et al. Thermodynamics of Palladium Dissolution Behavior in FetO–SiO2–CaO–Al2O3–MgO Slag at 1873 K. Metall Mater Trans B (2024). https://doi.org/10.1007/s11663-024-03127-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11663-024-03127-w