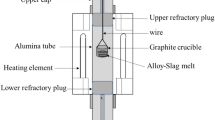
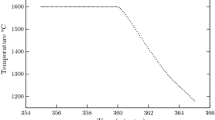
Abstract
Slag refining of molten Si–Fe alloy was investigated with the ultimate goal of producing silicon suitable for solar cell application. Multiple compositions of quaternary slag of CaO–Al2O3–SiO2–Na2O were used to treat Si-20 wt pct Fe alloy at 1300 °C. Partition ratio of B increased with increasing the slag basicity, and the maximum partition ratio of boron was found to be 4.3. The addition of 10 wt pct Na2O results in higher removal of boron. The modified quasichemical model was utilized to compute the activity of silica at 1300 °C which was then used to calculate the borate and phosphate capacities of the slag. A comparative thermodynamic assessment was conducted by establishing a relationship between corrected optical basicity and borate and phosphate capacities. The evaluation showed that lower temperature is favorable for increasing borate and phosphate capacities in slag at particular basicity.









Similar content being viewed by others
References
G.M. Wilson: J. Phys. D, 2020, vol. 53(49), p. 493001.
A.J. Trappey: J. Clean. Prod., 2016, vol. 112, pp. 1709–16.
Photovoltaics Report: Fraunhofer Institute for Solar Energy Systems ISE (2022 February). https://www.ise.fraunhofer.de/en/publications/studies/photovoltaics-report.html. Accessed 28 March 2022.
B.S. Xakalashe and M. Tangstad: Chem. Technol., 2012. pp. 32–37.
D. Helmreich and G. Ast: J. Electrochem. Soc. Meeting St. Louis, 1980, vol. 127, p. 111.
R. Hopkin, R.G. Seidensticker, J.R. Davis, P. Rai-Choudhury, P.D. Blais, and J.R. McCormick: J. Cryst. Growth, 1977, vol. 42, pp. 493–98.
J. Davis, A. Rohatgi, R.H. Hopkins, P.D. Blais, P. Rai-Choudhury, J.R. Mccormick, and H.C. Mollenkopf: IEEE Trans. Electron Devices, 1980, vol. 27(4), pp. 677–87.
F.A. Trumbore: Bell Syst. Tech. J., 1960, vol. 39(1), pp. 205–33.
E. Øvrelid, B. Geerligs, A. Wærnes, O. Raaness, I. Solheim, R. Jensen, and B. Wiersma: Proceedings Silicon for the Chem. Ind. VIII, 2006.
M. De Wild-Scholten and E. Alsema: Refocus, 2004, vol. 5(5), pp. 46–49.
S. Pizzini: Advanced Silicon Materials for Photovoltaic Applications, 1st ed. Wiley, New York, 2012, pp. 31–35.
A.F.B. Braga, S.P. Moreira, P.R. Zampieri, J.M.G. Bacchin, and P.R. Mei: Sol. Energy Mater. Sol. Cells, 2008, vol. 92(4), pp. 418–24.
B. Sørensen: Renewable Energy: Renewable Energy Origins and Flows, 1st ed. Routledge, London, 2018, pp. 182–88.
E. Williams: The Case of High-Purity Silicon and its App. in IT and Renew. Energy, United Nation University, Tokyo, Japan. 2000.
L. J. Geerligs, G. P. Wyers, R. Jensen, O. Raaness, A. N. Waernes, S. Santén, and B. Wiersma: 12th Works. Cryst. Silicon Sol. Cell Mater. and Process., NREL National Renewable Energy Laboratory, Breckenridge, CO, August 2002. pp. 215–18.
L. Ma, Z. Yu, W. Ma, S. Qing, and J. Wu: Silicon, 2019, vol. 11, pp. 1383–91.
C. Wagner: Metall. Trans. B, 1975, vol. 6(3), pp. 405–09.
L.A.V. Teixeira: ISIJ Int., 2009, vol. 49(6), pp. 777–82.
K. Morita: Treatise on Process Metallurgy, 2014. pp. 587–615.
S. Tabuchi and N. Sano: Metall. Trans. B, 1984, vol. 15(2), pp. 351–56.
J.J. Wu, Y.L. Li, K.X. Wei, Y.A.N.G. Bin, and Y.N. Dai: Trans. Nonferr. Met. Soc. China, 2014, vol. 14(4), pp. 1231–36.
J. Safarian, G. Tranell, and M. Tangstad: Metall Mater. Trans. B, 2013, vol. 44B, pp. 571–83.
X. Ma, M. Yoshikawa, and K. Morita: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 528–33.
R. Al-khazraji, Y.Q. Li, and L.F. Zhang: Int. J. Miner. Metall. Mater., 2018, vol. 25(12), pp. 1439–46.
L. Zhang, Y. Tan, F.M. Xu, J.Y. Li, H.Y. Wang, and Z. Gu: Sep. Sci. Technol., 2013, vol. 48(7), pp. 1140–44.
M. Johnston and M. Barati: Sol. Energy Mater. Sol. Cells, 2010, vol. 94(12), pp. 2085–90.
J. Li, L. Zhang, Y. Tan, D. Jiang, D. Wang, and Y. Li: Vacuum, 2014, vol. 103, pp. 33–37.
T. Yoshikawa and K. Morita: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 731–36.
T. Yoshikawa and K. Morita: ISIJ int., 2005, vol. 45(7), pp. 967–71.
T. Yoshikawa and K. Morita: ISIJ Int., 2007, vol. 47(4), pp. 582–84.
T. Yoshikawa, A. Arimura, and K. Morita: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 837–42.
T. Yoshikawa and K. Morita: Sci. Tech. Adv. Mater., 2003, vol. 4(6), pp. 531–37.
L. Hu, Z. Wang, X. Gong, Z. Guo, and H. Zhang: Sep. Purif. Technol., 2013, vol. 118, pp. 699–703.
C.H. Lu, M. Fang, H.X. Lai, L.Q. Huang, J. Chen, J.T. Li, and X.T. Luo: Trans. Tech. Publ. Ltd., 2013, vol. 690, pp. 962–66.
L.T. Khajavi, K. Morita, T. Yoshikawa, and M. Barati: J. Alloys Compd., 2015, vol. 619, pp. 634–38.
L.T. Khajavi and M. Barati: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 268–75.
L.T. Khajavi and M. Barati: High-Temp. Mater. Process., 2012, vol. 31(4–5), pp. 627–31.
S. Thomas, L. Huang, and M. Barati: JOM, 2021, vol. 73(1), pp. 260–81.
S. Thomas, M. Barati, and K. Morita: JOM, 2021, vol. 73(1), pp. 282–92.
A. Hosseinpour and L.T. Khajavi: J. Alloys Comp., 2018, vol. 768, pp. 545–52.
A. Hosseinpour and L.T. Khajavi: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 1773–81.
M. Li, T. Utigard, and M. Barati: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 221–28.
T.B. Massalski: Am. Soc. Met. Mater. Park, 1986, vol. 1, pp. 28–29.
G.I.A. Taposhe and L.T. Khajavi: JOM, 2021, vol. 73(2), pp. 729–35.
Y.Y. Hu, D.L. Lu, T. Lin, Y. Liu, B. Wang, C.J. Guo, and Q.S. Li: Adv. Mater. Res. Trans. Tech. Publ., 2011, vol. 156, pp. 882–85.
M. Tanahashi, Y. Shinpo, T. Fujisawa, and C. Yamauchi: Shiegn Sozai, 2002, vol. 118(7), pp. 497–505.
M. Li, T. Utigard, and M. Barati: Metall. Mater. Tans. B, 2015, vol. 46B, pp. 74–82.
I. Jung: Critical evaluation and thermodynamic modeling of phase equilibria in multicomponent oxide systems. PhD thesis, Ecole Polytechnique, Montreal, Canada, 2003.
A.D. Pelton and M. Blander: Calphad, 1988, vol. 12(1), pp. 97–108.
A.D. Pelton and M. Blander: Metall. Trans. B, 1986, vol. 17(4), pp. 805–15.
M. Blander and A.D. Pelton: Geochim. Cosmochim. Acta, 1987, vol. 51(1), pp. 85–95.
G. Eriksson, P. Wu, and A.D. Pelton: Calphad, 1993, vol. 17(2), pp. 189–205.
G. Eriksson and A.D. Pelton: Metall. Trans. B, 1993, vol. 24(5), pp. 807–16.
P. Chartrand and A.D. Pelton: Calphad, 1999, vol. 23(2), pp. 219–30.
J. Chipman, J.C. Fulton, N. Gokcen, and G.R. Caskey: Acta Metall., 1954, vol. 2(3), pp. 439–50.
A. Roine: Proceedings Int. Symposium on Computer Soft. Chem. Extractive Metallurgy. Pergamon, 1989.
J. Miettinen: Calphad, 1998, vol. 22(2), pp. 231–56.
R. Noguchi, K. Suzuki, F. Tsukihashi, and N. Sano: Metall. Mater. Trans. B, 1994, vol. 25, pp. 903–07.
A. Zaitsev, N.Y. Zaitseva, and A. Kodentsov: J. Mater. Chem., 2003, vol. 13(4), pp. 943–50.
D.R. Gaskell and D.E. Laughlin: Introduction to the Thermodynamics of Materials, 6 ed., vol. 53, CRC Press, Boca Raton, 2017, pp. 9363–67.
K.C. Mills: ISIJ Int., 1993, vol. 33(1), pp. 148–55.
Acknowledgments
The present work has been partially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2017-04669).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taposhe, G.I.A., Khajavi, L.T. Thermodynamics of Impurities Removal From Si–Fe Alloy by CaO–Al2O3–SiO2–Na2O Slag Refining. Metall Mater Trans B 53, 4019–4028 (2022). https://doi.org/10.1007/s11663-022-02662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02662-8