Abstract
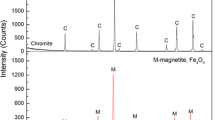
Fifteen commercial concentrates that are consumed in ferrochrome pre-oxidative sintering pelletizer operations were characterized, and exposed to oxidative roasting environments. Significant variance was found in the thermogravimetric behavior of the ores, which was observed to be strongly correlated with the starting Cr:Al ratio of the spinel (r = 0.84, P < 0.001). The formation of the sesquioxide phase during roasting was studied through comprehensive XRD and SEM–EDS analysis. Counter-diffusion of Mg2+ and Fe2+ cations with only limited diffusion of Cr3+ and Al3+ was observed. The final mass fraction of sesquioxide present after conversion was determined by calculations using SEM–EDS analyses and was found to be between 35 and 54 pct after oxidation at 1200 °C for 5 minutes. The mass fraction of sesquioxide was found to be most strongly correlated with the total Fe content of the starting chromite spinel (r = 0.93, P < 0.001). The sesquioxide phase that forms was confirmed to be a solid solution between Al2O3–Cr2O3–Fe2O3 with no evidence of pure hematite (Fe2O3) or eskolaite (Cr2O3) precipitate found.













Similar content being viewed by others
References
L. Holappa: in Handbook of Ferroalloys: Theory and Technology, 1st ed., M. Gasik, ed., Elsevier Science, Oxford, 2013, pp. 9–28.
ICDA: Ferrochrome Q1 2021 Quarterly Industry Overview, ICDA, 2021. https://www.icdacr.com/. Accessed 6 August 2021.
R. Fichte: in Handbook of Extractive Metallurgy, vol. III, F. Habashi, ed., Wiley-VCH, Weinheim, 1997, pp. 438–53.
R.T. Jones and M.W. Erwee: CALPHAD, 2016, vol. 55, pp. 20–25.
R. Urquhart: Miner. Sci. Eng., 1972, vol. 4, pp. 48–65.
E. Ringdalen, M. Rocha, and P. Figueiredo: Proc. 14th Int. Ferroalloys Conf., Kiev, Ukraine, 2015, pp. 668–75.
J.H. Zietsman, A. Steyn, and W. Pretorius: Proc. 15th Int. Ferroalloys Conf., Cape Town, South Africa, 2018, pp. 1–27.
L.A. Cramer, J. Basson, and L.R. Nelson: J. S. Afr. Inst. Min. Metall., 2004, vol. 104, pp. 517–27.
Outokumpu now Outotec: Australian Mining. https://www.australianmining.com.au/news/outokumpu-now-outotec/. Accessed 6 August 2021.
L. Hekkala, T. Fabritius, and J. Härkki: Proc. 10th Int. Ferroalloys Conf., Cape Town, South Africa, 2004, pp. 585–92.
Miningreview.com, G.: Metso Outotec Emerges from Merger, Miningreview.com, 2020. https://www.miningreview.com/international/metso-outotec-emerges-from-merger/. Accessed 6 August 2021.
J. Daavittila, M. Honkaniemi, and P. Jokinen: Proc. 10th Int. Ferroalloys Conf., Cape Town, South Africa, 2004, pp. 432–43.
R.J. Hill, J.R. Craig, and G.V. Gibbs: Phys. Chem. Miner., 1979, vol. 4, pp. 317–39.
L.W. Fisher: Am Mineral. J. Earth Planet. Mater., 1929, vol. 14, pp. 341–57.
Y.R. Murthy, S.K. Tripathy, and C.R. Kumar: Miner. Eng., 2011, vol. 24, pp. 375–80.
K. Perry, C. Finn, and R. King: Metall. Trans. B, 1988, vol. 19, pp. 677–84.
V. Urusov: Phys. Chem. Miner., 1983, vol. 9, pp. 1–5.
A. Hammarberg: Recovery of Valuable Metals or Metal Compounds from Complex Ores, United States Patent 2,123,240, 1938.
K. Konopicky and F. Caesar: Ber. Dtsch. Keram. Ges., 1939, vol. 20, pp. 367–73.
N.E. Dobbins and W.J. Rees: Special Report 32, The Iron and Steel Institute, London, 1946, pp. 211–33.
J.R. Rait: Third Report on Refractory Materials, The Iron and Steel Inst. Spec. Rept. No. 32, 1946, pp. 175–209.
A. Lekatou and D. Walker: Ironmak. Steelmak., 1995, vol. 22, pp. 227–38.
V.D. Tathavakar, M.P. Antony, and A. Jha: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 75–84.
B. Zhao and P.C. Hayes: Proc. 12th Int. Ferroalloys Conf., Helsinki, Finland, 2010, pp. 263–74.
J. Pan, C. Yang, and D. Zhu: ISIJ Int., 2015, vol. 55, pp. 727–35.
Y. **ao, M. Reuter, and L. Holappa: Proc. 9th Int. Ferroalloys Conf., Quebec City, Canada, 2001, pp. 147–56.
M.H. Khedr: ISIJ Int., 2000, vol. 40, pp. 309–14.
J.P. Beukes, N.F. Dawson, and P.G. Van Zyl: J. S. Afr. Inst. Min. Metall., 2010, vol. 110, pp. 743–50.
G. Ramakrishna, A. Kadrolkar, and N. Gurulaxmi Srikakulapu: Metall. Mater. Trans. B, 2014, vol. 46B, pp. 1073–81.
Y. Yang, Y. **ao, and M.A. Reuter: Proc. 10th Int. Ferroalloys Conf., Cape Town, South Africa, 2004, pp. 15–25.
E. Ringdalen: PhD Thesis, NTNU, Norway, 1999.
L.J. Erasmus: Proc. 7th Int. Ferroalloys Conf., Trondheim, Norway, 1995, pp. 273–85.
L.R. Nelson: Celebr. Megascale: Proc. Extr. Process. Div. Symp. Pyrometall. Honor of David G.C. Robertson, San Diego, California. 2014, pp. 39–68.
M.M. Langa, P.J. Jugo, M.I. Leybourne, D.F. Grobler, J. Adetunji, and H. Skogby: Miner. Depos., 2021, vol. 56, pp. 31–44.
C.J. Penberthy and R.K.W. Merkle: S. Afr. J. Geol., 1999, vol. 102, pp. 240–50.
G. Von Gruenewaldt, C.J. Hatton, and R.K.W. Merkle: Econ. Geol., 1986, vol. 81, pp. 1067–79.
P.A. Wagner: S. Afr. J. Sci., 1923, vol. 20, pp. 223–35.
C.R. Borra, G. Kapure, and V. Tathavadkar: Tata Search, 2010, vol. 1, pp. 145–48.
G. Kapure, V. Tathavadkar, C.B. Rao, S.M. Rao, and K.S. Raju: Proc. 12th Int. Ferroalloys Conf., Helsinki, Finland, 2010, pp. 293–302.
A. Biswas, B. Konar, G.U. Kapure, N. Sahu, and M. Paliwal: Miner. Process. Extr Metall., 2021, vol. 130, pp. 31–41.
D. Zhu, C. Yang, J. Pan, and X. Li: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 2919–30.
D. Zhu, C. Yang, J. Pan, Q. Zhang, B. Shi, and F. Zhang: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 1010–23.
E.L.J. Kleynhans, B.W. Neizel, J.P. Beukes, and P.G. Van Zyl: Miner. Eng., 2016, vol. 92, pp. 114–24.
V. Tathavadkar, M. Antony, and A. Jha: Scand. J. Metall., 2004, vol. 33, pp. 65–75.
M. Yildirim, D. Özyürek, and T. Tunçay: High Temp. Mater. Process., 2017, vol. 36, pp. 515–21.
H. Rietveld: Acta Crystallogr., 1967, vol. 22, pp. 151–52.
N. Doebelin and R. Kleeberg: J. Appl. Crystallogr., 2015, vol. 48, pp. 1573–80.
A. Vaitkus, A. Merkys, and S. Gražulis: J. Appl. Crystallogr, 2021, vol. 54, pp. 661–72.
R.T. Downs and M. Hall-Wallace: Am. Mineral., 2003, vol. 88, pp. 247–50.
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, and M.A. Van Ende: CALPHAD, 2016, vol. 54, pp. 35–53.
S.A. Degterov, A.D. Pelton, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 643–57.
M. Hillert: J. Alloys Compd., 2001, vol. 320, pp. 161–76.
Eastplats: Eastern Platinum Limited Reports Successful PGM Production from Scavenger Circuit and Operational Update, 18 Feb 2020. https://eastplats.com/eastern-platinum-limited-reports-successful-pgm-production-from-scavenger-circuit-and-operational-update/. Accessed 26 July 2021.
G.T.R. Droop: Mineral. Mag., 1987, vol. 51, pp. 431–35.
P.B. Arab, T.P. Araújo, and O.J. Pejon: Appl. Clay Sci., 2015, vol. 114, pp. 133–40.
M. Wesołowski: Thermochim. Acta, 1984, vol. 78, pp. 395–421.
B.Z. Dlugogorski and R.D. Balucan: Renew. Sustain. Energy Rev., 2014, vol. 31, pp. 353–67.
D.D. Howat, P.R. Jochens, J.C. Payntcr, and N.A. Barcza: Report No. 1365, National Institute for Metallurgy, 1972.
N.A. Barcza: Master's Thesis, University of Witwatersrand, South Africa, 1971.
S. Sánchez-Ramos, A. Doménech-Carbó, J.V. Gimeno-Adelantado, J. Peris-Vicente, and F.M. Valle-Algarra: Thermochim. Acta, 2008, vol. 476, pp. 11–19.
L. **ng, Y. Qu, C. Wang, L. Shao, Z. Zou, and W. Song: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 395–406.
J.M. Gallardo Amores, V. Sanchez Escribano, and G. Busca: Mater. Chem. Phys., 1999, vol. 60, pp. 168–76.
I. Kondofersky, A. Müller, H.K. Dunn, A. Ivanova, G. Stefanic, M. Ehrensperger, C. Scheu, B.A. Parkinson, D. Fattakhova-Rohlfing, and T. Bein: J. Am. Chem. Soc., 2016, vol. 138, pp. 1860–67.
J. Manjanna and G. Venkateswaran: Ind. Eng. Chem. Res., 2002, vol. 41, pp. 3053–63.
R.E. Carter, W.L. Roth, and C.A. Julien: J. Am. Ceram. Soc., 1959, vol. 42, pp. 533–36.
D. Hubble and N. Dodge: Am. Ceram. Soc. Bull., 1961, vol. 40, pp. 498–502.
W.S. Treffner: J. Am. Ceram. Soc., 1962, vol. 45, pp. 455–63.
G. Rigby, R. Hutton, and B. Hamilton: J. Am. Ceram. Soc., 1963, vol. 46, pp. 332–42.
M.D. Abràmoff, P.J. Magalhães, and S.J. Ram: Biophotonics Int., 2004, vol. 11, pp. 36–42.
A. Muan and S. Sömiya: J. Am. Ceram. Soc., 1959, vol. 42, pp. 603–13.
A.M. Suzuki, A. Yasuda, and K. Ozawa: Phys. Chem. Miner., 2008, vol. 35, pp. 433–45.
L. Chandrakantha: Using Excel Solver in Optimization Problems, Mathematics and Computer Science Department, John Jay College of Criminal Justice of CUNY, 2014, pp. 42–49.
J. Demšar, T. Curk, A. Erjavec, Č Gorup, T. Hočevar, M. Milutinovič, M. Možina, M. Polajnar, M. Toplak, and A. Starič: J. Mach. Learn. Res., 2013, vol. 14, pp. 2349–53.
J. Talar: Arch. Metall. Mater., 2007, vol. 52, pp. 239–50.
Acknowledgments
The authors would like to thank and acknowledge Samancor Chrome for financial assistance toward this research as well as supplying the ore samples. The authors would also like to thank Markus Erwee for assisting with the co-ordination and execution of experimental runs; Wiebke Grote for the XRD analysis; Archie Corfield for high quality sample mounting and polishing; and Carel Coetzee for gracious access to the SEM facilities.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swanepoel, S., Garbers-Craig, A.M. & De Villiers, J.P.R. The Oxidation Behavior of a Selection of South African Chromites. Metall Mater Trans B 53, 3805–3824 (2022). https://doi.org/10.1007/s11663-022-02643-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02643-x