Abstract
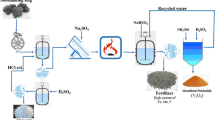
Conventional vanadium (V) extraction methods for vanadium-bearing slags create high chromium content discharge Cr(VI)-containing hazardous wastes. In this article, an ecofriendly magnesiation roasting-acid leaching method is proposed to selectively extract V from the slag. In the near-zero discharge process the generation of Cr(VI) is eliminated. Cr present as (Fe0.6Cr0.4)2O3 in the leach residue in association with Fe2O3 and SiO2 can be directly smelted to produce ferrochrome. From the obtained leach liquor, V, Mg and Mn are recovered successively. At pH 3.0, V precipitates as ammonium polyvanadate, which is calcined to V2O5 (99.14 pct pure) with a total recovery rate of 94.10 pct. Meanwhile, the NH3 gas produced during calcination is recycled for pH adjustment. At pH 8 to 9, Mn precipitates as hydroxide and is calcined to produce a MnO by-product (91.10 pct pure; recovery rate 47.96 pct). Subsequently, at pH 10 and above, dissolved Mg is precipitated as Mg(OH)2 and calcined to obtain MgO (purity = 99.12 pct; recovery = 90.47 pct). The calcine MgO is recycled as a roasting additive. The evaporation of residual solution yielded (NH4)2SO4 crystals and condensed water, which are recycled in vanadate precipitation and acid leaching, respectively.
Graphical abstract














Similar content being viewed by others
References
R.R. Moskalyk and A.M. Alfantazi: Miner. Eng., 2003, vol. 16, pp. 793–805.
H.Y. Li, Y. Yang, M. Zhang, W. Wei, and B. **e: J. Hazard. Mater., 2019, vol. 368, pp. 670–9.
S.Y. Chen, X.J. Fu, M.S. Chu, Z.G. Liu, and J. Tang: J. Clean. Prod., 2015, vol. 101, pp. 122–8.
B. Dhal, H.N. Thatoi, N.N. Das, and B.D. Pandey: J. Hazard Mater., 2013, vol. 250–251, pp. 272–91.
Y.M. Zhang, S.X. Bao, T. Liu, T.J. Chen, and J. Huang: Hydrometallurgy., 2011, vol. 109, pp. 116–24.
M. Karthikeyan and S. Um: J. Alloys Compd., 2017, vol. 695, pp. 1770–7.
X. Liang, G. Gao, Y. Liu, T. Zhang, and G. Wu: J. Alloys Compd., 2017, vol. 715, pp. 374–83.
H.-Y. Li, H.-X. Fang, K. Wang, W. Zhou, Z. Yang, X.-M. Yan, W.-S. Ge, Q.-W. Li, and B. **e: Hydrometallurgy., 2015, vol. 156, pp. 124–35.
H.-Y. Li, D. Li, Y. Guo, Y. Yang, W. Wei, and B. **e: Sens Actuators B., 2018, vol. 277, pp. 30–8.
J. Zhang, W. Zhang, L. Zhang, and S. Gu: Int. J. Miner. Process., 2015, vol. 138, pp. 20–9.
X.-S. Li, B. **e, G.-E. Wang, and X.-J. Li: Trans. Nonferrous Met. Soc. China., 2011, vol. 21, pp. 1860–7.
H.-Y. Li, K. Wang, W.-H. Hua, Z. Yang, W. Zhou, and B. **e: Hydrometallurgy., 2016, vol. 160, pp. 18–25.
T. Jiang, J. Wen, M. Zhou, and X. Xue: J. Alloys Compd., 2018, vol. 742, pp. 402–12.
J. Wen, T. Jiang, M. Zhou, H.-Y. Gao, J.-Y. Liu, and X.-X. Xue: Int. J. Min. Met. Mater., 2018, vol. 25, pp. 515–26.
B. Liu, H. Du, S.-N. Wang, Y. Zhang, S.-L. Zheng, L.-J. Li, and D.-H. Chen: AlChE J., 2013, vol. 59, pp. 541–52.
H.-Y. Li, C. Wang, M. Lin, Y. Guo, and B. **e: Powder Technol., 2020, vol. 360, pp. 503–8.
Y.L. Ji, S.B. Shen, J.H. Liu, and Y. Xue: J. Clean. Prod., 2017, vol. 149, pp. 1068–78.
H.B. Liu, H. Du, D.W. Wang, S.N. Wang, S.L. Zheng, and Y. Zhang: Trans. Nonferrous Met. Soc. China., 2013, vol. 23, pp. 1489–500.
J. Wen, T. Jiang, J. Wang, H. Gao, and L. Lu: J. Hazard. Mater., 2019, vol. 378, p. 120733.
J. Wen, T. Jiang, Y. Liu, and X. Xue: Miner. Process. Extr. Metal. Rev., 2019, vol. 40, pp. 56–66.
S.A. Katz and H. Salem: J. Appl. Toxicol., 1993, vol. 13, pp. 217–24.
J. Wen, T. Jiang, H. Gao, W. Zhou, Y. Xu, X. Zheng, Y. Liu, and X. Xue: J. Environ. Manag., 2019, vol. 244, pp. 119–26.
J. Wen, T. Jiang, Y. Xu, J. Cao, and X. Xue: J. Ind. Eng. Chem., 2019, vol. 71, pp. 327–35.
Y. Ji, S. Shen, J. Liu, S. Yan, and Z. Zhang: ACS Sustain. Chem. Eng., 2017, vol. 5, pp. 6008–15.
J. Wen, T. Jiang, Y. Xu, J. Liu, and X. Xue: Metall. MateR. Trans. B., 2018, vol. 49, pp. 1471–81.
J. Wen, T. Jiang, J. Wang, L. Lu, and H. Sun: J. Clean. Prod., 2020, vol. 261, p. 121205.
G. Wang, M.-M. Lin, J. Diao, H.-Y. Li, B. **e, and G. Li: ACS Sustain. Chem. Eng., 2019, vol. 7, pp. 18133–41.
J. Wen, T. Jiang, W.Y. Zhou, H.Y. Gao, and X.X. Xue: Sep. Purif. Technol., 2019, vol. 216, pp. 126–35.
J. Cheng, C. J. Wang, S. Shen, J. Diao, B. **e and H.-Y. Li, Jom, 2021, pp. 1–7.
H.-X. Fang, H.-Y. Li, and B. **e: ISIJ Int., 2012, vol. 52, pp. 1958–65.
J. Zhang, W. Zhang, and Z. Xue: Metals., 2019, vol. 9, p. 21.
H.-Y. Li, C.-J. Wang, Y.-H. Yuan, Y. Guo, J. Diao, and B. **e: J. Clean. Prod., 2020, vol. 260, p. 121091.
O. Pelletier, P. Davidson, C. Bourgaux, C. Coulon, S. Regnault, and J. Livage: Langmuir., 2000, vol. 16, pp. 5295–303.
R. Kumar and A.K. Biswas: Hydrometallurgy., 1986, vol. 15, pp. 267–80.
F.M. Capece, V. Di Castro, C. Furlani, G. Mattogno, C. Fragale, M. Gargano, and M. Rossi: J. Electron. Spectrosc. Relat. Phenom., 1982, vol. 27, pp. 119–28.
E. Desimoni, C. Malitesta, P.G. Zambonin, and J.C. Riviere: Surf. Interface Anal., 1988, vol. 13, pp. 173–9.
X.-S. Li and B. **e: Int. J. Min. Met. Mater., 2012, vol. 19, pp. 595–601.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 52074050); Chongqing Science and Technology Bureau (cstc2019jcyjjqX0006, cstc2021ycjh-bgzxm0075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted November 5, 2021; accepted November 23, 2021.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, HY., Cheng, J., Wang, CJ. et al. Ecofriendly Selective Extraction of Vanadium from Vanadium Slag with High Chromium Content via Magnesiation Roasting–Acid Leaching. Metall Mater Trans B 53, 604–616 (2022). https://doi.org/10.1007/s11663-021-02402-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02402-4