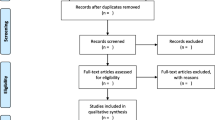
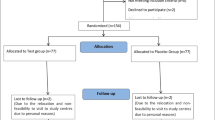
Abstract
Objective
To assess the efficacy and safety of mulberry twig alkaloids (Sangzhi alkaloids, SZ-A) for treatment of type 2 diabetes in a randomized, double-blind, placebo-controlled multicenter clinical trial.
Methods
A total of 200 patients were randomized to receive SZ-A (n=100) or placebo (n=100) for 16 weeks. The data analysis system for electronic data capture clinical trial central randomization system was used for randomization and dispensing of drugs. The primary outcome was the change in glycosylated hemoglobin (HbA1c) level. The secondary outcome included the proportions of cases with HbA1c <7.0% and HbA1c <6.5%, fasting blood glucose (FBG), postprandial blood glucose (PBG), area under curve for the PBG (AUC0-2h), body weight, and body mass index (BMI). Adverse events (AEs), severe adverse events (SAEs), treatment-related adverse events (TAEs), gastrointestinal disorders (GDs), blood pressure, routine blood tests, and liver and kidney function were monitored.
Results
Compared with baseline, the change of HbA1c at week 16 was −0.80% (95% CI: −0.98% to −0.62%) and −0.09% (95% CI: −0.27% to 0.09%) in SZ-A group and placebo group, respectively. The proportion of patients with HbA1c <7% and <6.5% was higher in the SZ-A group than in the placebo group (46.8% vs. 21.6% and 29.9% vs. 10.8%). The observed values and changes in FBG, 1 h-PBG, 2 h-PBG, and AUC0-2h differed significantly between groups (P<0.001), but differences were not significant in body weight and BMI (P>0.05). The incidence rates of AEs, TAEs, and GDs differed significantly between groups (P=0.010, P=0.005, and P=0.006, respectively), whereas the incidence rates of SAEs showed no significant differences between groups (P=1.000).
Conclusion
SZ-A are effective and safe for treatment of type 2 diabetes. The protocol was registered in http://www.chictr.org.cn/showproj.aspx?proj=60117 (ChiCTR2000038550)
Similar content being viewed by others
References
Diabetes Atlas, E.E.C., IDF Diabetes Atlas. 9th edition 2019. 2019.
Organization W.H. Global report on diabetes. WHO, 2016. 2016.
Mahboubi M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol Res 2019;146:104341.
Thaipitakwong T, Supasyndh O, Rasmi Y, Aramwit P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement Ther Med 2020;49:102292.
Ye F, Shen Z, Fand **e M. Alpha-glucosidase inhibition from a Chinese medical herb (Ramulus mori) in normal and diabetic rats and mice. Phytomedicine 2002;9:161–166.
Ye F, Shen ZF, Qiao FX, Zhao DY, **e MZ, et al. Experimental treatment of complications in alloxan diabetic rats with alpha-glucosidase inhibitor from the Chinese medicinal herb Ramulus mori. Acta Pharmaceut Sin 2002;37:108–112.
Liu SN, Liu Q, Sun SJ, Li CN, Huan Y, Chen YT, et al. Anti-diabetic effects of the fraction of alkaloids from Ramulus Mori, an innovative Sangzhi alkaloids as an α-glucosidase inhibitor. Acta Pharmaceut Sin 2019;7:1225–1233.
Qu L, Liang XC, Tian GQ, Zhang GL, Wu QL, Huang XM, et al. Efficacy and safety of Mulberry Twig Alkaloids Tablet for the treatment of type 2 diabetes: A multicenter, randomized, double-blind, double-dummy, and parallel controlled clinical trial. Diabetes Care 2021;44: 1324–1333.
Rodrigues EL, Marcelino G, Silva GT, Figueiredo PS, Garcez WS, Corsino J, et al. Nutraceutical and medicinal potential of the morus species in metabolic dysfunctions. Int J Mol Sci 2019;14;20:301.
Chan EW, Lye PY and Wong SK. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med 2016;14:17–30.
Zhang L, Tao GJ, Chen J, Zheng ZP. Characterization of a new flavone and tyrosinase inhibition constituents from the Twigs of Morus alba L. Molecules 2016;21:1130.
Yang S, Mi JQ, Liu ZH, Wang BL, **a XJ, Wang RY, et al. Pharmacokinetics, tissue distribution, and elimination of three active alkaloids in rats after oral administration of the effective fraction of alkaloids from ramulus mori, an innovative hypoglycemic agent. Molecules 2017;22:1616.
American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S90–S102.
Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, et al. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean diabetes association. Diabetes Metab J 2017;41:337–348.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract 2019;25:69–100.
Liu ZH, Yang Y, Dong WJ, Liu Q, Wang RY, Pang JM, et al. Investigation on the enzymatic profile of mulberry alkaloids by enzymatic study and molecular docking. Molecules 2019;24i:E1776.
Yang S, Wang BL, **a XJ, Li X, Wang RY, Sheng L, et al. Simultaneous quantification of three active alkaloids from a traditional Chinese medicine Ramulus mori (sangzhi) in rat plasma using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2015;109:177–183.
Gao K, Zheng CL, Wang T, Zhao HH, Wang J, Wang ZY, et al. 1-Deoxynojirimycin: occurrence, extraction, chemistry, oral pharmacokinetics, biological activities and in silico target fishing. Molecules 2021;21:1600.
Acknowledgments
The authors would like to thank Prof. ZHENG Qing-shan, Pharmaceutical Clinical Research Center of Shanghai University of Traditional Chinese Medicine, for his statistical support. In addition, we thank the participants, experimenters, and researchers for their participation. We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this manuscript. Prof. LIANG **ao-chun is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
Qu L wrote the manuscript and performed clinical research on the patients at the center. Liang XC and Tian GQ were responsible for the project design, coordination, article review, and clinical research. Liu YL and Shen ZF were responsible for drug development. Zhang GL, Wu QL, Huang XM, Cui YZ, Ma GQ, Lu H, Li Y, Jiang H, Yang XY, Zhang GD, and Yang CH were responsible for clinical research at their corresponding centers.
Corresponding author
Ethics declarations
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this study that could have influenced its outcome.
Additional information
Supported by the National Major Science and Technology Projects of China (No. 2013ZX09101005)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Qu, L., Liang, Xc., Tian, Gq. et al. Efficacy and Safety of Mulberry Twig Alkaloids Tablet for Treatment of Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Study. Chin. J. Integr. Med. 28, 304–311 (2022). https://doi.org/10.1007/s11655-021-2885-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-021-2885-9