Abstract
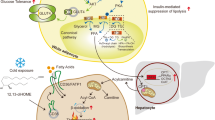
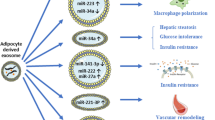
Excessive fat deposition in obese subjects promotes the occurrence of metabolic diseases, such as type 2 diabetes mellitus (T2DM), cardiovascular diseases, and non-alcoholic fatty liver disease (NAFLD). Adipose tissue is not only the main form of energy storage but also an endocrine organ that not only secretes adipocytokines but also releases many extracellular vesicles (EVs) that play a role in the regulation of whole-body metabolism. Exosomes are a subtype of EVs, and accumulating evidence indicates that adipose tissue exosomes (AT Exos) mediate crosstalk between adipose tissue and multiple organs by being transferred to targeted cells or tissues through paracrine or endocrine mechanisms. However, the roles of AT Exos in crosstalk with metabolic organs remain to be fully elucidated. In this review, we summarize the latest research progress on the role of AT Exos in the regulation of metabolic disorders. Moreover, we discuss the potential role of AT Exos as biomarkers in metabolic diseases and their clinical application.
Similar content being viewed by others
References
Tziomalos K, Athyros VG, Karagiannis A, et al. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis, 2010,20(2):140–146
Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol, 2011,11(2):85–97
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest, 2017,127(1):1–4
Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab, 2016,23(5):770–784
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003,112(12):1796–1808
Li Y, Soos TJ, Li X, et al. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem, 2004,279(44):45304–45307
Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb, 2011,18(8):629–639
Pekgor S, Duran C, Berberoglu U, et al. The Role of Visceral Adiposity Index Levels in Predicting the Presence of Metabolic Syndrome and Insulin Resistance in Overweight and Obese Patients. Metab Syndr Relat Disord, 2019,17(5):296–302
Chen Y, Pfeifer A. Brown Fat-Derived Exosomes: Small Vesicles with Big Impact. Cell Metab, 2017,25(4):759–760
Cocucci E. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol, 2015,25(6):364–372
Zhang Y, Shi L, Mei H, et al. Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutr Metab (Lond), 2015,12:21
Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature, 2017,542(7642):450–455
Zhao H, Shang Q, Pan Z, et al. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes, 2018,67(2):235–247
Ying W, Riopel M, Bandyopadhyay G, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell, 2017,171(2):372–384.e12
Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles, 2018,7(1):1535750
Martinez MC, Andriantsitohaina R. Extracellular Vesicles in Metabolic Syndrome. Circ Res, 2017,120(10):1674–1686
Malloci M, Perdomo L, Veerasamy M, et al. Extracellular Vesicles: Mechanisms in Human Health and Disease. Antioxid Redox Signal, 2019,30(6):813–856
Durcin M, Fleury A, Taillebois E, et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles, 2017,6(1):1305677
Bateman RM, Sharpe MD, Jagger JE, et al. 36th International Symposium on Intensive Care and Emergency Medicine: Brussels, Belgium. 15–18 March 2016. Crit Care, 2016,20(Suppl 2):94
Kranendonk ME, de Kleijn DP, Kalkhoven E, et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol, 2014,13:37
Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes, 2009,58(11):2498–2505
Lazar I, Clement E, Dauvillier S, et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res, 2016,76(14):4051–4057
Iacomino G, Russo P, Stillitano I, et al. Circulating microRNAs are deregulated in overweight/obese children: preliminary results of the I.Family study. Genes Nutr, 2016,11:7
Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol, 2013,9(9):513–521
Ortega FJ, Mercader JM, Moreno-Navarrete JM, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care, 2014,37(5):1375–1383
Amosse J, Durcin M, Malloci M, et al. Phenoty** of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of macrophage migration inhibitory factor. Mol Metab, 2018,18:134–142
Lee JE, Moon PG, Lee IK, et al. Proteomic Analysis of Extracellular Vesicles Released by Adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J, 2015,34(3):220–235
S ELA, Mager I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov, 2013,12(5):347–357
Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol, 2015,40:41–51
D’Souza-Schorey C, Schorey JS. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem, 2018,62(2):125–133
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci, 2018,75(2):193–208
Willms E, Cabanas C, Mager I, et al. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol, 2018,9:738
Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl), 2013,91(4):431–437
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol, 2018,19(4):213–228
Schmidt O, Teis D. The ESCRT machinery. Curr Biol, 2012,22(4):R116–R120
Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci, 2013,126(Pt 24):5553–5565
Hoshino D, Kirkbride KC, Costello K, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep, 2013,5(5):1159–1168
Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol, 2003,19:397–422
Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem, 2013,288(17):11649–11661
Lopez-Montero I, Monroy F, Velez M, et al. Ceramide: from lateral segregation to mechanical stress. Biochim Biophys Acta, 2010,1798(7):1348–1356
Elsherbini A, Bieberich E. Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy. Adv Cancer Res, 2018,140:121–154
Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases, 2018,9(1–2):95–106
Liu J, Zhang Y, Tian Y, et al. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics, 2022,12(3):1342–1372
Hartwig S, De Filippo E, Goddeke S, et al. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim Biophys Acta Proteins Proteom, 2019,1867(12):140172
Clement E, Lazar I, Attane C, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J, 2020,39(3):e102525
Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science, 2008,319(5867):1244–1247
Subra C, Grand D, Laulagnier K, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res, 2010,51(8):2105–2120
Gao Y, Qin Y, Wan C, et al. Small Extracellular Vesicles: A Novel Avenue for Cancer Management. Front Oncol, 2021,11:638357
Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol, 2007,212(1):174–181
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science, 2020,367(6478):eaau6977
Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, et al. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS One, 2015,10(9):e0138849
Zhang Y, Liu Y, Liu H, et al. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci, 2019,9:19
Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A, 2016,113(8):E968–E977
Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol, 2007,9(6):654–659
Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res, 2012,40(21):10937–10949
Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics, 2013,14:319
Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet, 2015,16(7):421–433
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 1993,75(5):843–854
Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol, 2019,20(1):5–20
Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci, 2011,68(16):2667–2688
Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol, 2006,Chapter 3:Unit 3.22
Laulagnier K, Javalet C, Hemming FJ, et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci, 2018,75(4):757–773
Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 2017,546(7659):498–503
Vargas A, Zhou S, Ethier-Chiasson M, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J, 2014,28(8):3703–3719
Escrevente C, Keller S, Altevogt P, et al. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer, 2011,11:108
Heusermann W, Hean J, Trojer D, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol, 2016,213(2):173–184
Prada I, Amin L, Furlan R, et al. A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. Biotechniques, 2016,60(1):35–41
Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem, 2014,289(32):22258–22267
Villarroya F, Cereijo R, Villarroya J, et al. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol, 2017,13(1):26–35
Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol, 2008,9(5):367–377
Crewe C, Joffin N, Rutkowski JM, et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell, 2018,175(3):695–708.e613
Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med, 2013,19(8):487–500
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol, 2006,6(10):772–783
Men Y, Yelick J, ** S, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun, 2019,10(1):4136
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem, 2019,88:487–514
Bebelman MP, Smit MJ, Pegtel DM, et al. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther, 2018,188:1–11
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest, 2016,126(4):1208–1215
Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol, 2019,21(1):9–17
Aoki N, **-no S, Nakagawa Y, et al. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology, 2007,148(8):3850–3862
Kranendonk ME, Visseren FL, van Balkom BW, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring), 2014,22(5):1296–1308
Connolly KD, Guschina IA, Yeung V, et al. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J Extracell Vesicles, 2015,4:29159
Huang XY, Chen JX, Ren Y, et al. Exosomal miR-122 promotes adipogenesis and aggravates obesity through the VDR/SREBF1 axis. Obesity (Silver Spring), 2022,30(3):666–679
Yao F, Yu Y, Feng L, et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARgamma of insulin resistance induced by high-fat diet-associated obesity. Exp Cell Res, 2017,355(2):105–112
Pan Y, Hui X, Hoo RLC, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest, 2019,129(2):834–849
Flaherty SE, 3rd, Grijalva A, Xu X, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science, 2019,363(6430):989–993
Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell, 2012,151(2):400–413
Nedergaard J, Golozoubova V, Matthias A, et al. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta, 2001,1504(1):82–106
Liu J, Wang Y, Lin L. Small molecules for fat combustion: targeting obesity. Acta Pharm Sin B, 2019,9(2):220–236
Chouchani ET, Kazak L, Spiegelman BM. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab, 2019,29(1):27–37
Lee YH, Petkova AP, Mottillo EP, et al. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab, 2012,15(4):480–491
Chen Y, Buyel JJ, Hanssen MJ, et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun, 2016,7:11420
Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia, 2016,59(5):879–894
Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology, 2018,155(4):407–417
Chen Y, Siegel F, Kipschull S, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun, 2013,4:1769
Ying W, Gao H, Dos Reis FCG, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab, 2021,33(4):781–790
An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif, 2021,54(3):e12993
Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol, 2017,13(11):674–686
Kakleas K, Soldatou A, Karachaliou F, et al. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev, 2015,14(9):781–797
Abdi R, Fiorina P, Adra CN, et al. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes, 2008,57(7):1759–1767
Volarevic V, Arsenijevic N, Lukic ML, et al. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells, 2011,29(1):5–10
Volarevic V, Al-Qahtani A, Arsenijevic N, et al. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity, 2010,43(4):255–263
Wang J, Yi Y, Zhu Y, et al. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo **u Fu Chong Jian Wai Ke Za Zhi (Chinese), 2020,34(1):124–131
Li X, **e X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med, 2018,50(4):1–14
Blazquez R, Sanchez-Margallo FM, de la Rosa O, et al. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol, 2014,5:556
Ma J, Zhang Z, Wang Y, et al. Investigation of miR-126-3p loaded on adipose stem cell-derived exosomes for wound healing of full-thickness skin defects. Exp Dermatol, 2022,31(3):362–374
Togliatto G, Dentelli P, Gili M, et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond), 2016,40(1):102–111
He L, Zhu C, Jia J, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/beta-catenin pathway. Biosci Rep, 2020,40(5):BSR20192549
Cooper DR, Wang C, Patel R, et al. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv Wound Care (New Rochelle), 2018,7(9):299–308
Wu YL, Lin ZJ, Li CC, et al. Adipose exosomal noncoding RNAs: Roles and mechanisms in metabolic diseases. Obes Rev, 2024,25(6):e13740
Zhang Y, Tian Z, Ye H, et al. Emerging functions of circular RNA in the regulation of adipocyte metabolism and obesity. Cell Death Discov, 2022,8(1):268
Song M, Han L, Chen FF, et al. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell Physiol Biochem, 2018,48(4):1416–1432
Tsai S, Clemente-Casares X, Revelo XS, et al. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? Diabetes, 2015,64(6):1886–1897
Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med, 2017,23(7):804–814
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol, 2019,70(3):531–544
Mziaut H, Henniger G, Ganss K, et al. MiR-132 controls pancreatic beta cell proliferation and survival through Pten/Akt/Foxo3 signaling. Mol Metab, 2020,31:150–162
Dusaulcy R, Handgraaf S, Visentin F, et al. miR-132-3p is a positive regulator of alpha-cell mass and is downregulated in obese hyperglycemic mice. Mol Metab, 2019,22:84–95
Cui X, You L, Zhu L, et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism, 2018,78:95–105
Setyowati Karolina D, Sepramaniam S, Tan HZ, et al. miR-25 and miR-92a regulate insulin I biosynthesis in rats. RNA Biol, 2013,10(8):1365–1378
Qian B, Yang Y, Tang N, et al. M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3beta/beta-catenin pathway in mice. Diabetologia, 2021,64(9):2037–2051
Cione E, Cannataro R, Gallelli L, et al. Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals (Basel), 2021,14(12):1257
Gesmundo I, Pardini B, Gargantini E, et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic beta cells. JCI Insight, 2021,6(5):e141962
Katayama M, Wiklander OPB, Fritz T, et al. Circulating Exosomal miR-20b-5p Is Elevated in Type 2 Diabetes and Could Impair Insulin Action in Human Skeletal Muscle. Diabetes, 2019,68(3):515–526
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology, 2010,51(2):679–689
Li D, Song H, Shuo L, et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY), 2020,12(22):22719–22743
Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res, 2014,192(2):268–275
Eguchi A, Lazic M, Armando AM, et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl), 2016,94(11):1241–1253
Heneghan HM, Miller N, McAnena OJ, et al. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab, 2011,96(5):E846–E850
Argyropoulos C, Wang K, McClarty S, et al. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One, 2013,8(1):e54662
Barutta F, Tricarico M, Corbelli A, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One, 2013,8(11):e73798
de Gonzalo-Calvo D, van der Meer RW, Rijzewijk LJ, et al. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep, 2017,7(1):47
Deng L, Huang Y, Li L, et al. Serum miR-29a/b expression in gestational diabetes mellitus and its influence on prognosis evaluation. J Int Med Res, 2020,48(9):300060520954763
Eissa S, Matboli M, Aboushahba R, et al. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications, 2016,30(8):1585–1592
Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother, 2016,83:92–99
Wan S, Wang J, Wang J, et al. Increased serum miR-7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res Clin Pract, 2017,130:171–179
Flowers E, Aouizerat BE, Abbasi F, et al. Circulating microRNA-320a and microRNA-486 predict thiazolidinedione response: Moving towards precision health for diabetes prevention. Metabolism, 2015,64(9):1051–1059
Karolina DS, Tavintharan S, Armugam A, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab, 2012,97(12):E2271–E2276
Zbikowski A, Blachnio-Zabielska A, Galli M, et al. Adipose-Derived Exosomes as Possible Players in the Development of Insulin Resistance. Int J Mol Sci, 2021,22(14):7427
Li M, Ke QF, Tao SC, et al. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B, 2016,4(42):6830–6841
Tao SC, Rui BY, Wang QY, et al. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv, 2018,25(1):241–255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This work was supported by the National Natural Science Foundation of China (No. 82070859).
Rights and permissions
About this article
Cite this article
He, R., Chen, Y. The Role of Adipose Tissue-derived Exosomes in Chronic Metabolic Disorders. CURR MED SCI 44, 463–474 (2024). https://doi.org/10.1007/s11596-024-2902-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-024-2902-2