Abstract
Objective
Abnormal expression of T-lymphokine-activated killer cell-originated protein kinase (TOPK) was reported to be closely related to the resistance of prostate cancer to radiotherapy and to targeted drug resistance in lung cancer. However, the role of TOPK inhibition in enhancing radiosensitivity of colorectal cancer (CRC) cells is unclear. This study aimed to evaluate the radiosensitization of TOPK knockdown in CRC cells.
Methods
The expression of TOPK was detected in CRC tissues by immunohistochemistry, and the effect of TOPK knockdown was detected in CRC cells by Western blotting. CCK-8 and clonogenic assays were used to detect the growth and clonogenic ability of CRC cells after TOPK knockdown combined with radiotherapy in CRC cells. Furthermore, proteomic analysis showed that the phosphorylation of TOPK downstream proteins changed after radiotherapy. DNA damage was detected by the comet assay. Changes in the DNA damage response signaling pathway were analyzed by Western blotting, and apoptosis was detected by flow cytometry.
Results
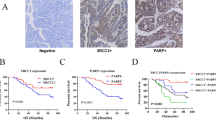
The expression of TOPK was significantly greater in CRC tissues at grades 2–4 than in those at grade 1. After irradiation, CRC cells with genetically silenced TOPK had shorter comet tails and reduced expression levels of DNA damage response-associated proteins, including phospho-cyclin-dependent kinase 1 (p-CDK1), phospho-ataxia telangiectasia-mutated (p-ATM), poly ADP-ribose polymerase (PARP), and meiotic recombination 11 homolog 1 (MRE11).
Conclusions
TOPK was overexpressed in patients with moderately to poorly differentiated CRC. Moreover, TOPK knockdown significantly enhanced the radiosensitivity of CRC cells by reducing the DNA damage response.
Similar content being viewed by others
References
Chen B, Alarado DM, Iticovici M, et al. Interferon-Induced IDO1 Mediates Radiation Resistance and Is a Therapeutic Target in Colorectal Cancer. Cancer Immunol Res, 2020,8(4):451–464
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021,71(3):209–249
** Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol, 2021,14(10):101174
Park SY, Lee CJ, Choi JH, et al. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res, 2019,38(1):399
Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet, 2019,394(10207):1467–1480
Siegel RL, MillerI KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin, 2019,69(1):7–34
Gaudet S, Branton D, Lue RA. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A, 2000,97(10):5167–5172
Abe Y, Matsumoto S, Kito K, et al. Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J Biol Chem, 2000,275(28):21525–21531
Herbert KJ, Ashton TM, Prevo R, et al. T-LAK cell-originated protein kinase (TOPK): an emerging target for cancer-specific therapeutics. Cell Death Dis, 2018,9(11):1089
Park JH, Lin ML, Nishidate T, et al. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res, 2006,66(18):9186–9195
Brown-clay JD, Shenoy DN, Timofeeva O, et al. PBK/TOPK enhances aggressive phenotype in prostate cancer via beta-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion. Oncotarget, 2015,6(17):15594–15609
Ohashi T, Komastu S, Ichikawa D, et al. Overexpression of PBK/TOPK relates to tumor malignant potential and poor outcome of gastric carcinoma. Br J Cancer, 2016,116(2):218–226
Ohashi T, Komatsu S, Ichikawa D, et al. Overexpression of PBK/TOPK Contributes to Tumor Development and Poor Outcome of Esophageal Squamous Cell Carcinoma. Anticancer Res, 2016,36(12):6457–6466
Qiao L, Ba J, **e J, et al. Overexpression of PBK/TOPK relates to poor prognosis of patients with breast cancer: a retrospective analysis. World J Surg Oncol, 2022,20(1):316
Simons-evelyn M, Bailey-dell K, Toretsky JA, et al. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. J Blood Cells Mol Dis, 2001,27(5):825–829
Zhu F, Zykova TA, Kang BS, et al. Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology, 2007,133(1):219–231
Zykova TA, Zhu F, Wang L, et al. The T-LAK Cell-originated Protein Kinase Signal Pathway Promotes Colorectal Cancer Metastasis. EBioMedicine, 2017,18:73–82
Li Y, Yang Z, Li W, et al. TOPK promotes lung cancer resistance to EGFR tyrosine kinase inhibitors by phosphorylating and activating c-Jun. Oncotarget, 2016,7(6):6748–6764
Kruthika BS, Jain R, Arivazhagan A, et al. Transcriptome profiling reveals PDZ binding kinase as a novel biomarker in peritumoral brain zone of glioblastoma. J Neurooncol, 2018,141(2):315–325
Mao P, Bao G, Wang YC, et al. PDZ-Binding Kinase-Dependent Transcriptional Regulation of CCNB2 Promotes Tumorigenesis and Radio-Resistance in Glioblastoma. Transl Oncol, 2020,13(2):287–294
Ma H, Qi G, Han F, et al. PBK drives PARP inhibitor resistance through the TRIM37/NFkappaB axis in ovarian cancer. Exp Mol Med, 2022,54(7):999–1010
Park JH, Park SA, Lee YJ, et al. PBK attenuates paclitaxel-induced autophagic cell death by suppressing p53 in H460 non-small-cell lung cancer cells. FEBS Open Bio, 2020,10(5):937–950
**ao JJ, Wang F, Lu H, et al. Targeting the COX2/MET/TOPK signaling axis induces apoptosis in gefitinib-resistant NSCLC cells. Cell Death Dis, 2019,10(10):777
Pirovano G, Ashton TM, Herbert KJ, et al. TOPK modulates tumour-specific radiosensitivity and correlates with recurrence after prostate radiotherapy. Br J Cancer, 2017,117(4):503–512
Ame JC, Spenlehauer C, Murcia GD. The PARP superfamily. Bioessays, 2004,26(8):882–893
Bharti SK, Brosh RM. Fine-tuning DNA repair by protein acetylation. Cell Cycle, 2016,15(15):1952–1953
Carney JP, Maser RS, Olivares H, et al. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell, 1998,93(3):477–486
Habraken Y, Jolois O, Piette J. Differential involvement of the hMRE11/hRAD50/NBS1 complex, BRCA1 and MLH1 in NF-kappaB activation by camptothecin and X-ray. Oncogene, 2003,22(38):6090–6099
Kobayashi J. Molecular mechanism of the recruitment of NBS1/hMRE11/hRAD50 complex to DNA double-strand breaks: NBS1 binds to gamma-H2AXthrough FHA/BRCT domain. J Radiat Res, 2004,45(4):473–47
Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev, 2001,15(17):2177–2196
Khosravi R, Maya R, Gottlieb T, et al. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci U S A, 1999,96(26):14973–14977
Wang XJ, Chu HY, Lv MJ, et al. Structure of the intact ATM/ Tel1 kinase. Nature Communications, 2016,7:11655
** MH, Oh DY. ATM in DNA repair in cancer. Pharmacol Ther, 2019,203:107391
Wang YQ, Yang LF, Zhang J, et al. Radiosensitization by irinotecan is attributed to G2/M phase arrest, followed by enhanced apoptosis, probably through the ATM/Chk/Cdc25C/Cdc2 pathway in p53-mutant colorectal cancer cells. Int J Oncol, 2018,53(4):1667–1680
Wang Z, Lai ST, Ma NY, et al. Radiosensitization of metformin in pancreatic cancer cells via abrogating the G2 checkpoint and inhibiting DNA damage repair. Cancer Lett, 369(1):192–201
Matsuo Y, Park JH, Miyamoto T, et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Transl Med,2014,6(259):259ra145
Herbert KJ, Puliyadi R, Prevo R, et al. Targeting TOPK sensitises tumour cells to radiation-induced damage by enhancing replication stress. Cell Death Differ, 2021,28(4):1333–1346
Mirza-aghazadeh-attari M, Darband SG, Kaviani M, et al. DNA damage response and repair in colorectal cancer: Defects, regulation and therapeutic implications. DNA Repair (Amst),2018,69:34–52
Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther, 2020,5(1):60
Deng S, Vlatkovic T, Li MY, et al. Targeting the DNA Damage Response and DNA Repair Pathways to Enhance Radiosensitivity in Colorectal Cancer. Cancers (Basel), 2022,14(19):4874
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This study was supported by the Guangxi Zhuang Autonomous Region Program of China (No. Z-C20220797), Guangxi Science and Technology Planning Project of China (No. Guike AD20297047), and National Natural Science Foundation of China (No. 81902849).
Rights and permissions
About this article
Cite this article
Pang, Sg., Zhang, X., Li, Zx. et al. TOPK Inhibition Enhances the Sensitivity of Colorectal Cancer Cells to Radiotherapy by Reducing the DNA Damage Response. CURR MED SCI 44, 545–553 (2024). https://doi.org/10.1007/s11596-024-2884-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-024-2884-0