Abstract
Objective
Brain microvascular endothelial cells (BMECs) were found to shift from their usually inactive state to an active state in ischemic stroke (IS) and cause neuronal damage. Ginsenoside Rb1 (GRb1), a component derived from medicinal plants, is known for its pharmacological benefits in IS, but its protective effects on BMECs have yet to be explored. This study aimed to investigate the potential protective effects of GRb1 on BMECs.
Methods
An in vitro oxygen-glucose deprivation/reperfusion (OGD/R) model was established to mimic ischemia-reperfusion (I/R) injury. Bulk RNA-sequencing data were analyzed by using the Human Autophagy Database and various bioinformatic tools, including gene set enrichment analysis (GSEA), Gene Ontology (GO) classification and enrichment analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, protein-protein interaction network analysis, and molecular docking. Experimental validation was also performed to ensure the reliability of our findings.
Results
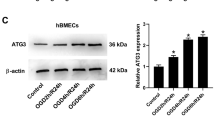
Rb1 had a protective effect on BMECs subjected to OGD/R injury. Specifically, GRb1 was found to modulate the interplay between oxidative stress, apoptosis, and autophagy in BMECs. Key targets such as sequestosome 1 (SQSTM1/p62), autophagy related 5 (ATG5), and hypoxia-inducible factor 1-alpha (HIF-1α) were identified, highlighting their potential roles in mediating the protective effects of GRb1 against IS-induced damage.
Conclusion
GRbl protects BMECs against OGD/R injury by influencing oxidative stress, apoptosis, and autophagy. The identification of SQSTM1/p62, ATG5, and HIF-1α as promising targets further supports the potential of GRb1 as a therapeutic agent for IS, providing a foundation for future research into its mechanisms and applications in IS treatment.
Similar content being viewed by others
References
Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J, 2015,282(21):4067–4079
Sweeney MD, Zhao Z, Montagne A, et al. Blood-brain barrier: From physiology to disease and back. Physiol Rev, 2019,99(1):21–78
Wong BW, Marsch E, Treps L, et al. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J, 2017,36(15):2187–2203
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med, 2013,19(12):1584–1596
Gong L, Yin J, Zhang Y, et al. Neuroprotective mechanisms of ginsenoside rb1 in central nervous system diseases. Front Pharmacol, 2022,13:914352
Hwang JY, Shim JS, Song MY, et al. Proteomic analysis reveals that the protective effects of ginsenoside rb1 are associated with the actin cytoskeleton in β-amyloid-treated neuronal cells. J Ginseng Res, 2016,40(3):278–284
Jiang N, Wang K, Zhang Y, et al. Protective effect of ginsenoside rb1 against chronic restraint stress (CRS)-induced memory impairments in rats. Behav Brain Res, 2021,405:113146
Jiang Z, Wang Y, Zhang X, et al. Preventive and therapeutic effects of ginsenoside rb1 for neural injury during cerebral infarction in rats. Am J Chin Med, 2013,41(2):341–352
Ni XC, Wang HF, Cai YY, et al. Ginsenoside rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol, 2022,54:102363
Li DW, Zhou FZ, Sun XC, et al. Ginsenoside rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res, 2019,14(10):1814–1822
Orellana-Urzúa S, Rojas I, Líbano L, et al. Pathophysiology of ischemic stroke: Role of oxidative stress. Curr Pharm Des, 2020,26(34):4246–4260
Stark R, Grzelak M, Hadfield J. RNA sequencing: The teenage years. Nat Rev Genet, 2019,20(11):631–656
Thind AS, Monga I, Thakur PK, et al. Demystifying emerging bulk RNA-seq applications: The application and utility of bioinformatic methodology. Brief Bioinform, 2021,22(6):bbab259
Gong L, Zhang D, Dong Y, et al. Integrated bioinformatics analysis for identificating the therapeutic targets of aspirin in small cell lung cancer. J Biomed Inform, 2018,88:20–28
Gauthier J, Vincent AT, Charette SJ, et al. A brief history of bioinformatics. Brief Bioinform, 2019,20,(6):1981–1996
Tomkins JE, Manzoni C. Advances in protein-protein interaction network analysis for parkinson’s disease. Neurobiol Dis, 2021,155:105395
Ferreira LG, Dos Santos RN, Oliva G, et al. Molecular docking and structure-based drug design strategies. Mol Basel Switz, 2015,20(7):13384–13421
Pinzi L, Rastelli G. Molecular docking: Shifting paradigms in drug discovery. Int J Mol Sci, 2019,20(18):4331
Zhou C, Zhong W, Zhou J, et al. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy, 2012,8(8):1215–1226
O’Boyle NM, Banck M, James CA, et al. Open babel: An open chemical toolbox. J Cheminformatics, 2011,3:33
Eberhardt J, Santos-Martins D, Tillack AF, et al. AutoDock vina 1.2.0: New docking methods, expanded force field, and python bindings. J Chem Inf Model, 2021,61(8):3891–3898
Elefantova K, Lakatos B, Kubickova J, et al. Detection of the mitochondrial membrane potential by the cationic dye JC-1 in l1210 cells with massive overexpression of the plasma membrane ABCB1 drug transporter. Int J Mol Sci, 2018,19(7):1985
Cossarizza A, Baccarani-Contri M, Kalashnikova G, et al. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolc arbocyanine iodide (JC-1). Biochem Biophys Res Commun, 1993,197(1):40–45
Perelman A, Wachtel C, Cohen M, et al. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis, 2012,3(11):e430
Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. JAMA, 2021,325(11):1088–1098
Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol, 2018,315(3):C343–C356
Ornatowski W, Lu Q, Yegambaram M, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol, 2020,36:101679
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell, 2008,132(1):27–42
Li ZY, Wu YF, Xu XC, et al. Autophagy as a double-edged sword in pulmonary epithelial injury: A review and perspective. Am J Physiol Lung Cell Mol Physiol, 2017,313(2):L207–L217
Filomeni G, Desideri E, Cardaci S, et al. Under the ROS: Thiol network is the principal suspect for autophagy commitment. Autophagy, 2010,6(7):999–1005
Larson-Casey JL, He C, Carter AB. Mitochondrial quality control in pulmonary fibrosis. Redox Biol, 2020,33:101426
Yang N, Guan QW, Chen FH, et al. Antioxidants targeting mitochondrial oxidative stress: Promising neuroprotectants for epilepsy. Oxid Med Cell Longev, 2020,2020:6687185
Zhang L, Wang K, Lei Y, et al. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med, 2015,89:452–465
Fu ZJ, Wang ZY, Xu L, et al. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol, 2020,36:101671
Ichimura Y, Waguri S, Sou Y-S, et al. Phosphorylation of p62 activates the keap1-nrf2 pathway during selective autophagy. Mol Cell, 2013,51(5):618–631
Basit F, van Oppen LM, Schöckel L, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis, 2017,8(3):e2716
Qin X, Zhang J, Wang B, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy, 2021,17(12):4266–4285
Schütt F, Aretz S, Auffarth GU, et al. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Invest Ophthalmol Vis Sci, 2012,53(9):5354–5361
Yang NC, Jeng KCG, Ho WM, et al. ATP depletion is an important factor in DHEA-induced growth inhibition and apoptosis in BV-2 cells. Life Sci, 2002,70(17):1979–1988
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
On behalf of all the authors, the corresponding author states that there is no conflict of interests.
Additional information
This study was funded by the Science and Technology Innovation Project of the China Academy of Chinese Medical Sciences (Nos. CI2021A04618 and CI2021A01401).
Rights and permissions
About this article
Cite this article
Luo, Ym., Liu, Ss., Zhao, M. et al. Crosstalk among Oxidative Stress, Autophagy, and Apoptosis in the Protective Effects of Ginsenoside Rb1 on Brain Microvascular Endothelial Cells: A Mixed Computational and Experimental Study. CURR MED SCI 44, 578–588 (2024). https://doi.org/10.1007/s11596-024-2858-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-024-2858-2