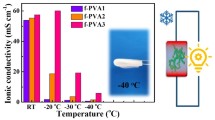
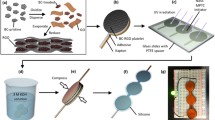
Abstract
The lifetime and application of electrochemical storage devices are always threatened by thermal runaway. Intelligent self-protecting gel electrolytes can be designed using temperature-responsive polymers. However, the mechanisms and factors affecting protective behavior are unclear. Here, we fabricated supercapacitors using temperature-responsive polyacrylamide-2-hydroxyethyl acrylate (PNIPAM-co-HEA) hydrogel polyelectrolytes. It was found that the polymer changed from hydrophilic to hydrophobic with increasing temperature, and the physical cross-linking of the polymer molecular strands in the electrolyte was enhanced, thus restricting conductive ion migration and closing the ion transportation pathway. The hydrophilic–hydrophobic transition on the gel surface also contributed to the suppression of the specific capacitance of the supercapacitor. This self-protection feature is repeatable. In addition, we investigated the effect of methyl groups in the main chain structure on the electrochemical properties using poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate) (PNIPAM-co-HEMA). Methylene enhanced the hydrophobicity of the polymer at room temperature and reduced the thermo-protective effect. The methyl group in the main chain also reduced the thermal response temperature of the polymer. This study explores the mechanism by which temperature-responsive polymers inhibit thermal runaway in supercapacitors and provides support for the design of more rational and efficient temperature-sensitive electrolytes.







Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Liang C, Wang S, Sha S, Lv S, Wang G, Wang B, Li Q, Yu J, Xu X, Zhang L (2023) Novel semiconductor materials for advanced supercapacitors. J Mater Chem C 11(13):4288–4317. https://doi.org/10.1039/d2tc04816g
Choi C, Ashby DS, Butts DM, DeBlock RH, Wei Q, Lau J, Dunn B (2020) Achieving high energy density and high power density with pseudocapacitive materials. Nat Rev Mater 5(1):5–19. https://doi.org/10.1038/s41578-019-0142-z
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energ Environ Sci 7(5):1597–1614. https://doi.org/10.1039/c3ee44164d
Rana S, Kumar R, Bharj RS (2023) Current trends, challenges, and prospects in material advances for improving the overall safety of lithium-ion battery pack. Chem Eng J 463:142336. https://doi.org/10.1016/j.cej.2023.142336
Lamba P, Singh P, Singh P, Singh P, Bharti, Kumar A, Gupta M, Kumar Y (2022) Recent advancements in supercapacitors based on different electrode materials: classifications, synthesis methods and comparative performance. J Energy Storage 48:103871. https://doi.org/10.1016/j.est.2021.103871
Najib S, Erdem E (2019) Current progress achieved in novel materials for supercapacitor electrodes: mini review. Nanoscale Adv 1(8):2817–2827. https://doi.org/10.1039/c9na00345b
Poonam, Sharma K, Arora A, Tripathi SK (2019) Review of supercapacitors: materials and devices. J Energy Storage 21:801–825. https://doi.org/10.1016/j.est.2019.01.010
Salleh NA, Kheawhom S, Hamid NAA, Rahiman W, Mohamad AA (2023) Electrode polymer binders for supercapacitor applications: a review. J Mater Res Technol 23:3470–3491. https://doi.org/10.1016/j.jmrt.2023.02.013
Gan L, Chen R, Yu X, Li H (2022) Understanding the battery safety improvement enabled by a quasi-solid-state battery design. Chinese Phys B 31:11820211. https://doi.org/10.1088/1674-1056/ac9221
Huang W, Feng X, Han X, Zhang W, Jiang F (2021) Questions and answers relating to lithium-ion battery safety issues. Cell Rep Phys Sci 2:1002851. https://doi.org/10.1016/j.xcrp.2020.100285
Xu G, Wang X, Ludi, Jang M, Huang S, Shangguan X, Cui G (2018) Research progress of high safety flame retardant electrolytes for lithium-ion batteries. Energy Storage Sci Technol 7(6):1040–1059. https://doi.org/10.12028/j.issn.2095-4239.2018.0153
Kaliaperumal M, Dharanendrakumar MS, Prasanna S, Abhishek KV, Chidambaram RK, Adams S, Zaghib K, Reddy MV (2021) Cause and mitigation of lithium-ion battery failure-a review. Materials 14:567619. https://doi.org/10.3390/ma14195676
Zhao R, Liu J, Gu J (2016) Simulation and experimental study on lithium ion battery short circuit. Appl Energ 173:29–39. https://doi.org/10.1016/j.apenergy.2016.04.016
Mallick S, Gayen D (2023) Thermal behaviour and thermal runaway propagation in lithium-ion battery systems-a critical review. J Energy Storage 62:106894. https://doi.org/10.1016/j.est.2023.106894
Li M, Chen Z (2021) Thermo-responsive polymers for thermal regulation in electrochemical energy devices. J Polym Sci. https://doi.org/10.1002/pol.20210433
Chan CY, Wang Z, Jia H, Ng PF, Chow L, Fei B (2021) Recent advances of hydrogel electrolytes in flexible energy storage devices. J Mater Chem A Mater Energy Sustain 9(4):243–269. https://doi.org/10.1039/d0ta09500a
Yang H, Leow WR, Chen X (2018) Thermal-responsive polymers for enhancing safety of electrochemical storage devices. Adv Mater 30(13):1704347. https://doi.org/10.1002/adma.201704347
Kim YG, Lee CH, Bae YC (2014) Hydrophilic-hydrophobic copolymer nano-sized particle gels: swelling behavior and dependence on crosslinker chain length. Fluid Phase Equilibr 361:200–207. https://doi.org/10.1016/j.fluid.2013.11.004
Shen Z, Terao K, Maki Y, Dobashi T, Ma G, Yamamoto T (2006) Synthesis and phase behavior of aqueous poly(N-isopropylacrylamide-co-acrylamide), poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) and poly (N-isopropylacrylamide-co-2-hydroxyethyl methacrylate). Colloid Polym Sci 284(9):1001–1007. https://doi.org/10.1007/s00396-005-1442-y
Gong C, Shi S, Wu L, Gou M, Yin Q, Guo Q, Dong P, Zhang F, Luo F, Zhao X, Wei Y, Qian Z (2009) Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel. Part 2: Sol–gel–sol transition and drug delivery behavior. Acta Biomater 5(9):3358–3370. https://doi.org/10.1016/j.actbio.2009.05.025
Ma G, Miao B, Song C (2010) Thermosensitive PCL-PEG-PCL hydrogels: synthesis, characterization, and delivery of proteins. J Appl Polym Sci. https://doi.org/10.1002/app.31654 (NA-NA)
Zhang C, Chen W, Hong Y, Wang X (2021) Surface activity and structure of temperature-responsive polymer surfactants based on PNIPAm at the air/solution interface. Langmuir 37(15):4632–4638. https://doi.org/10.1021/acs.langmuir.1c00320
Tian X, Yi Y, Fang B, Yang P, Wang T, Liu P, Qu L, Li M, Zhang S (2020) Design strategies of safe electrolytes for preventing thermal runaway in lithium ion batteries. Chem Mater 32(23):9821–9848. https://doi.org/10.1021/acs.chemmater.0c02428
Morimoto N, Yamamoto M (2021) Design of an LCST–UCST-like thermoresponsive Zwitterionic copolymer. Langmuir 37(11):3261–3269. https://doi.org/10.1021/acs.langmuir.0c03128
Zhou H, Liu H, Li Y, Yue X, Wang X, Gonzalez M, Meng YS, Liu P (2019) In situ formed polymer gel electrolytes for lithium batteries with inherent thermal shutdown safety features. J Mater Chem A 7(28):16984–16991. https://doi.org/10.1039/C9TA02341K
Yang Y, Yu D, Wang H, Guo L (2017) Smart electrochemical energy storage devices with self-protection and self-adaptation abilities. Adv Mater 29(45):1703040. https://doi.org/10.1002/adma.201703040
Kelly JC, Gupta R, Roberts ME (2015) Responsive electrolytes that inhibit electrochemical energy conversion at elevated temperatures. J Mater Chem A Mater Energy Sustain 3(7):426–434. https://doi.org/10.1039/c4ta06482h
Mo F, Li H, Pei Z, Liang G, Ma L, Yang Q, Wang D, Huang Y, Zhi C (2018) A smart safe rechargeable zinc ion battery based on sol-gel transition electrolytes. Sci Bull 63(16):1077–1086. https://doi.org/10.1016/j.scib.2018.06.019
Shi Y, Ha H, Al Sudani A, Ellison CJ, Yu G (2016) Thermoplastic elastomer-enabled smart electrolyte for thermoresponsive self-protection of electrochemical energy storage devices. Adv Mater 28(36):7921–7928. https://doi.org/10.1002/adma.201602239
**e B, Chen S, Chen Y, Liu L (2021) Self-shutdown function induced by sandwich-like gel polymer electrolytes for high safety lithium metal batteries. RSC Adv 11(23):14036–14046. https://doi.org/10.1039/D1RA02641K
Zhu J, Yao M, Huang S, Tian J, Niu Z (2020) Thermal-gated polymer electrolytes for smart zinc-ion batteries. Angew Chem Int Ed 59(38):16480–16484. https://doi.org/10.1002/anie.202007274
Zhang P, Wang J, Sheng W, Wang F, Zhang J, Zhu F, Zhuang X, Jordan R, Schmidt OG, Feng X (2018) Thermoswitchable on-chip microsupercapacitors: one potential self-protection solution for electronic devices. Energy Environ Sci 11(7):1717–1722. https://doi.org/10.1039/C8EE00365C
Kelly JC, Pepin M, Huber DL, Bunker BC, Roberts ME (2012) Reversible control of electrochemical properties using thermally-responsive polymer electrolytes. Adv Mater 24(7):886–889. https://doi.org/10.1002/adma.201103340
Yang H, Liu Z, Chandran BK, Deng J, Yu J, Qi D, Li W, Tang Y, Zhang C, Chen X (2015) Self-protection of electrochemical storage devices via a thermal reversible sol-gel transition. Adv Mater 27(37):5593–5598. https://doi.org/10.1002/adma.201502484
Haq MA, Su Y, Wang D (2017) Mechanical properties of PNIPAM based hydrogels: a review. Mater Sci Eng C-Mater Biol Appl 70(1):842–855. https://doi.org/10.1016/j.msec.2016.09.081
Liu J, Jiang L, He S, Zhang J, Shao W (2022) Recent progress in PNIPAM-based multi-responsive actuators: a mini-review. Chem Eng J 433:1334962. https://doi.org/10.1016/j.cej.2021.133496
Xu X, Liu Y, Fu W, Yao M, Ding Z, Xuan J, Li D, Wang S, **a Y, Cao M (2020) Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers-Basel 12:5803. https://doi.org/10.3390/polym12030580
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51873147).
Author information
Authors and Affiliations
Contributions
J.L. performed the investigation, data curation, formal analysis and writing (original Draft).
S.M. performed the conceptualization and writing (Review & Editing).
X.X. performed the supervision, project administration and writing (Review & Editing).
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Ma, S. & Xu, X. Study of temperature-sensitive gel electrolytes for energy storage devices with self-protection behavior. Ionics (2024). https://doi.org/10.1007/s11581-024-05580-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05580-8