Abstract
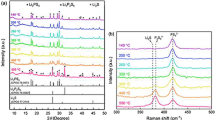
This study focuses on the preparation of lithium diphosphate compound LiCeP2O7 via conventional solid-state reaction. Analysis using the Rietveld refinement of the X-ray diffraction pattern reveals that the sample adopts a monoclinic structure at room temperature. Surface morphology is further examined through SEM. Additionally, complex impedance and electrical modulus spectroscopy analyses indicate the presence of non-Debye-type relaxation behavior. The direct current (dc) conductivity exhibits Arrhenius behavior, with activation energies of 0.94 eV in region I and 1.21 eV in region II, indicating thermally activated lithium-ion conduction. The thermal behavior of the exponent parameter “s” suggests a transition in the conduction mechanism from large polaron tunneling to small polaron tunneling that occurs at 533 K. Electric modulus studies confirm that the ionic conduction relaxation process is thermally activated and exhibits a spread of relaxation time. Understanding the ionic conduction mechanism will facilitate the design of efficient ionic conductors for battery applications.














Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Sadaba I, Lima S, Valente AA, Granados ML (2011) Catalytic dehydration of xylose to furfural: vanadyl pyrophosphate as source of active soluble species. Carbohydr Res 346:2785–2791
Fukuoka H, Matsunaga H, Yamanaka S (2003) Crystal structure and ionic conductivity of ruthenium diphosphate ARu2(P2O7)2, A=Li, Na, and Ag, with a tunnel structure. Mater Res Bullet 38:991–1001
Ternane R, Ferid M, Guyot Y, Trabelsi-Ayadi M, Boulon G (2008) Spectroscopic properties of Yb3+ in NaYbP2O7 diphosphate single crystals. J Alloys Compd 464:327–331
Llusar M, Garcıa A, Gargori C, Galindo R, Badenes JA, Monros G (2012) Synthesis of diphosphate Mn2−xMgxP2O7 solid solutions with thortveitite structure: new pink ceramic dyes for the colouration of ceramic glazes. J Eur Ceram Soc 32:765–776
Llusar M, Zielinska A, Tena MA, Badenes JA, Monros G (2010) Blue-violet ceramic pigments based on co and mg Co2−xMgxP2O7 diphosphates. J Eur Ceram Soc 30:1887–1896
Nishimura S, Nakamura N, Natsui R, Yamada A (2010) New lithium iron pyrophosphate as 3.5 V class cathode material for lithium ion battery. J Am Chem Soc 132(39):13596–13597
Wang S, Jiang X, Du G, Guo Z, Jang J, Kim S-J (2011) Solvothermal synthesis of Mn2P2O7 and its application in lithium-ion battery. Mater Lett 65:3265–3268
Sato T, Saito M, Sato C, Yin S (2009) Morphology control of cerium phosphates via solution processes for new sunscreen application. Funct Mater Lett 2(04):157–161
Ram R, Bhattacharya S (2023) Mixed ionic-electronic transport in Na2O doped glassy electrolytes: promising candidate for new generation sodium ion battery electrolytes. J Appl Phys 133:145101
Halder P, Bhattacharya S (2023) Debye to non-debye type relaxation in MoO3 doped glassy semiconductors: a portrait on microstructure and electrical transport properties. Phys B Condens Matter 648:414374
Sengupta A, Chamuah A, Ram R, Ghosh CK, Diyali S, Biswas B, Ali MS, Bhattacharya S (2022) Formation of Li10Zn4O9, Li2MoO3, and ZnSeO3 nanophases: roles in electrical conductivity and electrochemical stability in lithium ion conductors and their crystalline counterparts. ECS J Solid State Sci Technol 11:113008
Erragh F, Boukhari A, Sadel A, Holt EM (1998) Disodium zinc pyrophosphate and disodium (europium) zinc pyrophosphate. Acta Crystallogr Sect C: Cryst Struct Commun 54:1373–1376
Belharouak I, Gravereau P, Parent C, Chaminade JP, Lebraud E, Le Flem G (2000) Crystal structure of Na2ZnP2O7: reinvestigation. J Solid State Chem 152:466–473
Ghosh J, Sengupta A, Halder P, Ojha S, Panda GK, Bhattacharya S (2022) Single polaron hop** in Fe doped glassy semiconductors: structure–electrical transport relationship. J Appl Phys 132:205102
Bar AK, Bhattacharya K, Kundu R, Roy D, Bhattacharya S (2017) Anomalous electrical conductivity in selenite glassy nanocomposites. Mater Chem Phys 199:322–328
Bhattacharya S, Ghosh A (2007) Silver molybdate nanoparticles, nanowires, and nanorods embedded in glass nanocomposites. Phys Rev B 75:092103
El Maadi A, Boukhari A, Holt EM (1995) Crystal structures of the new diphosphates, K2NiP2O7 and K6Sr2Ni5(P2O7)5. J Chem Crystallogr 25:531–536
Laligant Y (1992) Structure determination of Na2PdP2O7 from X-ray powder diffraction. European J Solid State Inorg Chem 29:83–94
Jansen M, Wu GQ, Konigstein K, Krist Z (1991) Crystal structure of caesium ytterbium diphosphate, CsYbP2O7. Zeitschrift für Kristallographie-Crystalline Materials 197:245–246
Hamady A, Zid MF, Jouini T (1994) Structure cristalline de KYP2O7. J Solid State Chem 113:120–124
Hamady A, Jouini T (1996) K2MoO2 (MoO2As2O7)2, Acta Crystallographica section C: crystal structure. Communications 52(12):2947–2949
Jouini A, Gâcon JC, Férid M, Trabelsi-Ayadi M (2003) Luminescence and scintillation properties of praseodymium poly and diphosphates. Opt Mater 24:175–180
Férid M, Horchani K, Amami J (2004) Preparation, structure and infrared spectrum of NaEuP2O7. Mater Res Bull 39:1949–1955
Hamrit F, Chtourou R, Taloub D, Gharbi I, Oueslati A (2023) Synthesis, morphological, electrical, and conduction mechanism studies of a sodium cerium diphosphate compound. RSC Adv 13:15356–15365
Béjaoui A, Horchani-Naifer K, Hajji M, Férid M (2014) Crystal structure, physical properties and bond valence analysis of NaLuP2O7. Solid State Sci 31:46–53
Férid M, Horchani-Naifer K (2004) Synthesis, crystal structure and vibrational spectra of a new form of diphosphate NaLaP2O7. Mater Res Bull 39:2209–2217
Kumar U, Upadhyay S (2020) Structural, microstructure, optical, and dielectric properties of Sr1.99M0.01SnO4 (M: La, Nd, Eu) Ruddlesden–popper oxide. J Mater Sci Mater Electron 31:5721–5730
Béjaouin A, Horchani-Naifer K, Férid M (2013) Ionic conduction, bond valence analysis of structure–property relationships of NaHoP2O7. J Solid State Chem 204:224–232
Horchani K, Ferid M (2015) Structure and ionic conductivity of NaCeP2O7. Solid State Ion 176(23–24):1949–1953
Durif A (2013) Crystal chemistry of condensed phosphates. Springer Science & Business Media
Nasri S, Oueslati A, Chaabane I, Gargouri M (2016) AC conductivity, electric modulus analysis and electrical conduction mechanism of RbFeP O ceramic compound. Ceram Int 42:14041–14048
Cheema H, Kumar S, Alvi PA, Choudhary BL, Kumar U (2020) Synthesis and physical properties of nanopowder and electrical properties of bulk samples of ZnFe2-xNixO4 (x: 0, 0.05, 0.10). Adv Powder Technol 31:4241–4252
Kumar U, Upadhyay S (2021) Parvez Ahmad Alvi, study of reaction mechanism, structural, optical and oxygen vacancy-controlled luminescence properties of Eu-modified Sr2SnO4 Ruddlesden popper oxide. Phys B Condens Matter 604:412708
Sassi M, Oueslati A, Moutia N, Khirouni K, Gargouri M (2017) A study of optical absorption and dielectric properties in lithium chromium diphosphate compound. Ionics 23:847–855
Ajili O, Louati B, Guidara K (2018) Dielectric and ac ionic conductivity investigation of Li2SrP2O7. Indian J Phys 92:875–881
Khay N, Ennaciri A, Rulmont A (2001) Structure and vibrational spectra of double diphosphates TlLnP2O7 (Ln = Dy, Ho, Y, Er, Yb). Raman Spectroscop 32:1052–1058
Pu Y, Dong Z, Zhang P, Wu Y, Zhao J, Luo Y (2016) Dielectric, complex impedance and electrical conductivity studies of the multiferroic Sr2FeSi2O7 crystallized glass-ceramics. Alloys Compd 672:64–71
Smari M, Rahmouni H, Elghoul N, Walha I, Dhahria E, Khirouni K (2015) Electric–dielectric properties and complex impedance analysis of La0.5Ca0.5xAgxMnO3 manganites. RSC Adv 5:2177–2184
Kumar U, Upadhyay S (2021) Parvez Ahmad Alvi, study of reaction mechanism, structural, optical and oxygen vacancy-controlled luminescence properties of Eu-modified Sr2SnO4 Ruddlesden popper oxide. Phys B Condens Matter 604:412708
Nadeem M, Akhtar MJ, Haque MN (2008) Increase of grain boundary resistance with time by impedance spectroscopy in La0. 50Ca0. 50MnO3+ δ at 77 K. Solid State Commun 145:263–266
Shah M, Nadeem M, Idrees M, Atif M, Akhtar MJ (2013) Change of conduction mechanism in the impedance of grain boundaries in Pr0.4Ca0.6MnO3. J Magn Magn Mater 332:61–66
Jemaï R, Lahouli R, Hcini S, Rahmouni H, Khirouni K (2017) Investigation of nickel effects on some physical properties of magnesium based ferrite. J Alloys Compd 705:340–348
Sen S, Choudhary RNP, Pramanik P (2007) Structural and electrical properties of Ca2+−modified PZT electroceramics. Phys B Condens Matter 387:56–62
Ye H, Sun CQ, Huang H, Hing P (2001) Single semicircular response of dielectric properties of diamond films. Thin Solid Films 381(1):52–56
Ye H, Sun CQ, Hung H, Hing P (2001) Dielectric transition of nanostructured diamond films. Appl Phys Lett 78(13):1826–1828
Okutan M, Basaran E, Bakan HI, Yakuphanoglu F (2005) AC conductivity and dielectric properties of Co-doped TiO2. Phys B Condens Matter 364:300–305
Oueslati A (2017) Li+ ion conductivity and transport properties of LiYP2O7 compound. Ionics 23:857–867
Sassi M, Oueslati A, Moutia N, Khirouni K, Gargouri M (2017) A study of optical absorption and dielectric properties in lithium chromium diphosphate compound. Ionics 23:847–855
Chowdari BVR, Gopalakrishnan R (1987) AC conductivity analysis of glassy silver iodomolybdate system. Solid State Ionics 23:225–233
Pant M, Kanchan DK, Gondaliya N (2009) Transport properties and relaxation studies in BaO substituted Ag2O–V2O5–TeO2 glass system. Mater Chem Phys 115:98–104
Oueslati A, Hlel F, Guidara K, Gargouri M (2010) AC conductivity analysis and dielectric relaxation behavior of [N(C3H7)4]2Cu2Cl6. J Alloys Compd 492:508–514
Yadav V, Cheema H, Maurya RS, Kumar S, Alvi PA, Sharma M, Kumar U (2022) Study of structural, optical, dielectric, and electric properties of homovalently substituted Ce in SrTiO3 perovskite oxide. Ionics 28:5513–5524
Šalkus T, Kežionis A, Kazakevičius E, Dindune A, Kanepe Z, Ronis J, Orliukas AF (2010) Preparation and characterization of Li2.9Sc1.9−yYyZr0.1(PO4)3 (where y= 0, 0.1) solid electrolyte ceramics. Phase Transit 83:581–594
Yaroslavtsev AB, Stenina IA (2006) Complex phosphates with the NASICON structure (MxA2(PO4)3). Russ J Inorg Chem 51:S97–S116
Nasri S, Megdiche M, Guidara K, Gargouri M (2014) Electrical conductivity and dielectric relaxation behavior of AgFeP2O7 compound. Ionics 20:399–407
Elliot SR (1987) J Adv Phys 36:135
Ghosh A (1990) Frequency-dependent conductivity in bismuth-vanadate glassy semiconductors. Phys Rev B 41(3):1479
Chamuah A, Ojha S, Bhattacharya K, Ghosh CK, Bhattacharya S (2022) AC conductivity and electrical relaxation of a promising Ag2S-Ge-Te-Se chalcogenide glassy system. J Phys Chem Solids 166:110695
Ojha S, Roy M, Bhattacharya S (2022) Description of AC conductivity via Meyer-Neldel rule: a comparative study between oxide and chalcogenide systems. J Non-Cryst Solids 577:121307
Chamuah A, Bhattacharya K, Ali MS, Ghosh CK, Chattopadhyay D, Bhattacharya S (2021) Density of states, DC conductivity and physical properties of Ag2S-Ge–Te–se chalcogenide glassy system. Appl Phys A 127:1–10
Mollah S, Som KK, Bose K, Chaudri BK (1993) AC conductivity in Bi4Sr3Ca3CuyOx (y= 0–5) and Bi4Sr3Ca3− z LizCu4Ox (z= 0.1–1.0) semiconducting oxide glasses. J Appl Phys 74(2):931–937
Ojha S, Ali MS, Roy M, Bhattacharya S (2021) Hop** frequency and conductivity relaxation of promising chalcogenides: AC conductivity and dielectric relaxation approaches. Mater Res Express 8:085203
Megdiche M, Perrin-Pellegrino C, Gargouri M (2014) Conduction mechanism study by overlap** large-polaron tunnelling model in SrNiP2O7 ceramic compound. J Alloys Compd 584:209–215
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2024-2924-03”.
Author information
Authors and Affiliations
Contributions
• Mohamed Hamdi was responsible for conceiving the research idea, designing the study, and securing the funding. He conducted data collection, performed statistical analyses, and drafted the manuscript.
• Mohamed Abu Shuheil contributed to the study design and participated in data collection.
• Abderrazak Oueslati provided expert guidance in the study design and methodology.
Corresponding author
Ethics declarations
Competing interests
.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamdi, M., Shuheil, M.A. & Oueslati, A. Synthesis, morphological, and ionic conductivity of a lithium cerium diphosphate compound. Ionics (2024). https://doi.org/10.1007/s11581-024-05516-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05516-2