Abstract
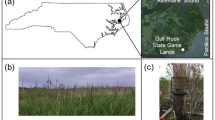
Matter and energy fluxes and ecosystem services in streams rely on fungi associated with leaf litter. However, the species diversity of this microbial group may differ between a protected area and its surroundings. Thus, to understand how beta diversity may depend on the conservation status of an area, we aimed to evaluate the structure and diversity (α, β, and γ) of the aquatic fungal community from a subtropical area and how it is distributed in streams from a protected area and from a farmland area. Hence, we compared eight streams inside with eight streams outside of a protected area. This is one of the first studies testing the biotic homogenisation hypothesis for fungi associated with leaf litter in streams. We found new records of fungal species from a global biodiversity hotspot. The gamma diversity of aquatic fungi was surprisingly high. Our analyses indicated a process of environmental homogenisation of streams outside the national park. Intriguingly, neither α nor β diversity differed between areas. Environmental variables presented large differences, especially regarding nutrients, which influence the aquatic fungal community. Altogether, this national park seems to be effective in protecting the environmental heterogeneity of streams to some extent; however, the national park was likely suffering from pollution, which requires further investigation.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akridge RE, Koehn RD (1987) Amphibious hyphomycetes from the San Marcos river in Texas. Mycologia 79:228–233
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Zeitschrift 22:711–728
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Anderson MJ, Walsh DCI (2013) PERMANOVA, ANOSIM and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83:557–574
Barbosa FR, Silva SS, Fiuza PO, Gusmão LFP (2011) Conidial fungi from the semi-arid Caatinga biome of Brazil, new species and records for Thozetella. Mycotaxon 115:327–334
Bärlocher F (1985) The role of fungi in the nutrition of stream invertebrates. Bot J Linn Soc 91:83–94
Bärlocher F (2000) Water-borne conidia of aquatic hyphomycetes: seasonal and yearly patterns in Catamaran Brook, New Brunswick, Canada. Canad J Bot 78:157–167
Bärlocher F, Boddy L (2016) Aquatic fungal ecology - how does it differ from terrestrial? Fungal Ecol 19:5–13
Bärlocher F, Rosset J (1981) Aquatic hyphomycetes spora of two black forest and two Swiss Jura streams. Trans Br Mycol Soc 76:479–483
Barros J, Seena S (2022) Fungi in freshwaters: prioritising aquatic hyphomycetes in conservation goals. Water 14:1–16
Baudoin JM, Guérold F, Felten V, Chauvet E, Wagner P, Rousselle P (2008) Elevated aluminium concentration in acidified headwater streams lowers aquatic hyphomycete diversity and impairs leaf-litter breakdown. Microb Ecol 56:260–269
Baudy P, Zubrod JP, Konschak M, Röder N, Nguyen TH, Schreiner VC, Baschien C, Schulz R, Bundschuh M (2021) Environmentally relevant fungicide levels modify fungal community composition and interactions but not functioning. Environ Pollut 285:117234
Biasi C, Graça MAS, Santos S, Ferreira V (2017) Nutrient enrichment in water more than in leaves affects aquatic microbial litter processing. Oecologia 184:555–568
Biasi C, Fontana LE, Restello RM, Hepp LU (2020) Effect of invasive Hoveniadulcis on microbial decomposition and diversity of hyphomycetes in Atlantic forest streams. Fungal Ecol 44:100890
Brasil (2006) Decreto de 23 de março de 2006. Cria o Parque Nacional dos Campos Gerais, no Estado do Paraná, e dá outras providências. Publicado no Diário Oficial da União de 24 de março de 2006. Available online at http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/dnn/Dnn10796.htm#:~:text=DNN%2010796&text=DECRETO%20DE%2023%20DE%20MARÇO,que%20lhe%20confere%20o%20art. Accessed 01 Feb 2017
Cadastro Nacional de Unidades de Conservação (CNUC) do Ministério do Meio Ambiente (2018). Available online at http://www.mma.gov.br/areas-protegidas/cadastro-nacional-de-ucs. Accessed 01 Feb 2018
Czeczuga B, Orłowska M (1997) Hyphomycetes fungi in rainwater falling from building roofs. Mycoscience 38:447–450
Chan SY, Goh TK, Hyde KD (2000) Ingoldian fungi in Hong Kong. In: Hyde KD, Ho W-H, Pointing SB (eds) Aquatic mycology across the millennium. Fungal Diversity Press p, Hong Kong, pp 89–107
Chauvet E (1991) Aquatic hyphomycete distribution in South-Western France. J Biogeogr 18:699–706
Cornut J, Ferreira V, Gonçalves AL, Chauvet E, Canhoto C (2015) Fungal alteration of the elemental composition of leaf litter affects shredder feeding activity. Freshw Biol 60:1755–1771
Crawford RH, Carpenter SE, Harmon ME (1990) Communities of filamentous fungi and yeast in decomposing logs of Pseudotsuga menziesii. Mycologia 82:759–765
Cruz ACR, Gusmão LFP (2009) Fungos conidiais na Caatinga: espécies associadas ao folhedo. Acta Bot Bras 23:999–1012
Cruz ACR, Gusmão LFP (2009) Fungos conidiais na Caatinga: espécies lignícolas. Acta Bot Bras 23:1133–1144
Cruz ACR, Marques MFO, Gusmão LFP (2007) Fungos anamórficos (Hyphomycetes) da Chapada Diamantina: novos registros para o Estado da Bahia e Brasil. Acta Bot Bras 21:847–855
Danger M, Gessner MO, Bärlocher F (2016) Ecological stoichiometry of aquatic fungi: current knowledge and perspectives. Fungal Ecol 19:100–111
Dudley N (2008) Guidelines for applying protected area management categories. IUCN, Gland Switzerland
Esteves FA, Gonçalves JFJr, (2011) Etapas do metabolismo aquático. In: Esteves FA (ed) Fundamentos de Limnologia. Interciência p, Rio de Janeiro, pp 119–124
Fellows CS, Valett HM, Dahm CN, Mulholland PJ, Thomas SA (2006) Coupling nutrient uptake and energy flow in headwater streams. Ecosystems 9:788–804
Fiuza PO, Gusmão LFP (2013) Ingoldian fungi from the semi-arid Caatinga biome of Brazil. Mycosphere 4:559–565
Fiuza PO, Ottoni-Boldrini BMP, Monteiro JS, Catena NR, Hamada N, Gusmão LFP (2015) First records of Ingoldian fungi from the Brazilian Amazon. Braz J Bot 38:615–621
Fiuza PO, Catillo-Pérez T, Gulis V, Gusmão LFP (2017) Ingoldian fungi of Brazil: some new records and a review including a checklist and a key. Phytotaxa 306:171–200
Gessner MO, Thomas M, Jean-Louis A-M, Chauvet E (1993) Stable successional patterns of aquatic hyphomycetes on leaves decaying in a summer cool stream. Mycol Res 97:163–172
Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K (2007) Fungal decomposers of plant litter in aquatic ecosystems. In: Kubicek CP, Druzhinina IS (eds) Environmental and microbial relationships. Springer p, Berlin, pp 301–324
Gibbons SM, Jones E, Bearquiver A, Blackwolf F, Roundstone W, Scott N, Hooker J, Madsen R, Coleman ML, Gilbert JA (2014) Human and environmental impacts on river sediment microbial communities. Plos One 9:1–9
Goh TK, Hyde KD (1996) Biodiversity of freshwater fungi. J Ind Microbiol Biotechnol 17:328–345
Gönczöl J, Csontos P, Révay A (2003) Catchment scale patterns of aquatic hyphomycetes: the role of physicochemical variables and substrate composition in structuring conidial communities. Arch Hydrobiol 157:249–266
Gönczöl J, Révay A (2002) Treehole fungal communities: aquatic, aero-aquatic and dematiaceous hyphomycetes. Fungal Divers 12:19–34
Graça MAS, Bärlocher F, Gessner MO (2005) Methods to study litter decomposition: a practical guide. Springer, Berlin
Graça MAS, Hyde K, Chauvet E (2016) Aquatic hyphomycetes and litter decomposition in tropical - subtropical low order streams. Fungal Ecol 19:182–189
Green JL, Holmes AJ, Westoby M, Oliver I, Briscoe D, Dangerfield M, Gillings M, Beattie AJ (2004) Spatial scaling of microbial eukaryote diversity. Nature 432:747–750
Gulis V (1999) Preliminary list of aquatic hyphomycetes from central Belarus. Mycotaxon 72:227–230
Heathwaite AL (2010) Multiple stressors on water availability at global to catchment scales: understanding human impact on nutrient cycles to protect water quality and water availability in the long term. Freshw Biol 55:241–257
Jabiol J, Bruder A, Gessner MO, Makkonen M, McKie BG, Peeters ETHM, Vos VCA, Chauvet E (2013) Diversity patterns of leaf-associated aquatic hyphomycetes along a broad latitudinal gradient. Fungal Ecol 6:439–448
Jackson DA (1993) Stop** rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence-absence data. J Animal Ecol 72:367–382
Krauss GJ, Solé M, Krauss G, Schlosser D, Wesenberg D, Bärlocher F (2011) Fungi in freshwaters: ecology, physiology and biochemical potential. FEMS Microbiol Rev 35:620–651
Kumar U, Nayak AK, Shahid M, Gupta VVSR, Panneerselvam P, Mohanty S, Kaviraj M, Kumar A, Chatterjee D, Lal B, Gautam P, Thipathi R, Panda BB (2018) Continuous application of inorganic and organic fertilizers over 47 years in paddy soil alters the bacterial community structure and its influence on rice production. Agric Ecosyst Environ 262:65–75
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116
Mathuriau C, Chauvet E (2002) Breakdown of leaf litter in a neotropical stream. J North Am Benthol Soc 21:384–396
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
Medeiros AO, Pascoal C, Graça MAS (2009) Diversity and activity of aquatic fungi under low oxygen conditions. Freshw Biol 54:142–149
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, Meiners T, Müller C, Obermaier E, Prati D, Socher SA, Sonnemann I, Wäschke N, Wubet T, Wurst S, Rillig MC (2014) Choosing and using diversity indices: insights for ecological applications from the German biodiversity exploratories. Ecol Evol 4:3514–3524
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2017) vegan: community ecology package. R package version 2.4-4. https://CRAN.R-project.org/package=vegan. Accessed 28 Oct 2017
Olden JD, Comte L, Giam X (2016) Biotic homogenisation. In eLS, John Wiley & Sons, Ltd, Chichester
Olden JD, Rooney TP (2006) On defining and quantifying biotic homogenization. Glob Ecol Biogeogr 15:113–120
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Oliveira EA (2012) O Parque Nacional dos Campos Gerais: processo de criação, caracterização ambiental e proposta de priorização de áreas para regularização fundiária. Setor de Ciências Agrárias da Universidade Federal do Paraná. p 297
Pascoal C, Marvanová L, Cássio F (2005) Aquatic hyphomycete diversity in streams of Northwest Portugal. Fungal Divers 19:109–128
Pattee E, Chergui H (1995) The application of habitat templets and traits to hyphomycete fungi in a mid-European river system. Freshw Biol 33:525–539
Petsch DK (2016) Causes and consequences of biotic homogenization in freshwater ecosystems. Int Rev Hydrobiol 101:113–122
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.r-project.org/. Accessed 01 Dec 2017
Rodrigues JLM, Pellizari VH, Mueller R, Baek K, Jesus EC, Paula FS, Mirza B, Hamaoui GS, Tsai SM, Feigl B, Tiedje JM, Bohannan BJM, Nüsslein K (2013) Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc Natl Acad Sci 110:988–993
Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanová L, Padgett D, Porter D, Raja HA, Schmit JP, Thorton HA, Voglymayr H (2007) Fungal biodiversity in aquatic habitats. Biodivers Conserv 16:49–67
Suberkropp K, Chauvet E (1995) Regulation of leaf breakdown by fungi in streams: influences of water chemistry. Ecology 76:1433–1445
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J North Am Benthol Soc 29:118–146
Tant CJ, Rosemond AD, Mehring AS, Kuehn KA, Davis JM (2015) The role of aquatic fungi in transformations of organic matter mediated by nutrients. Freshw Biol 60:1354–1363
Tuomisto H (2010) A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33:2–22
UNEP-WCMC and IUCN (2016) Protected Planet Report 2016. Cambridge UK and Gland Switzerland: UNEP-WCMC and IUCN. Available online on the website: https://resources.unep-wcmc.org/products/WCMC_RT062. Accessed 27 Jan 2018
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:280–338
Wong MKM, Goh T-K, Hodgkiss IJ, Hyde KD, Ranghoo VM, Tsui CKM, Ho W-H, Wong WSW, Yuen T-K (1998) Role of fungi in freshwater ecosystems. Biodivers Conserv 7:1187–1206
Wood-Eggenschwiler S, Bärlocher F (1985) Geographical distribution of Ingoldian fungi. Verh Internat Verein Limnol 22:2780–2785
Acknowledgements
The authors thank the Ecological Energetics Laboratory, Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (NUPELIA), Universidade Estadual de Ponta Grossa (UEPG), Universidade Tecnológica Federal do Paraná (UTFPR), Campos Gerais National Park, Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), Regiane Silva, Edivando Vitor do Couto, and Rachel Calil de Oliveira.
Funding
This work was supported by the Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná (FA) and Fundação Grupo Boticário de Proteção à Natureza (FGB) [grant number 45751 002/2016], the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [141691/2020-4 to M.M.R.S., 152643/2016-8 to G.D.P, and 308522/2021-4 to E.B.], and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [1614297 and 88881.690087/2022-01 to M.M.R.S.].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Matheus Maximilian Ratz Scoarize, Gisele Daiane Pinha, and Laryssa Helena Ribeiro Pazianoto. Analysis was performed by Matheus Maximilian Ratz Scoarize and Gisele Daiane Pinha. The first draft of the manuscript was written by Matheus Maximilian Ratz Scoarize, and all authors commented on previous versions of the manuscript. Funding acquisition was performed by Evanilde Benedito. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Claus Baessler
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scoarize, M.M.R., Pinha, G.D., Pazianoto, L.H.R. et al. Stream environmental conditions are homogenised outside a protected area, but fungal beta diversity remains unchanged. Mycol Progress 23, 12 (2024). https://doi.org/10.1007/s11557-024-01952-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01952-6