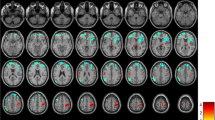
Abstract
Functional magnetic resonance imaging (fMRI) studies on migraine with aura are challenging due to the rarity of patients with triggered cases. This study optimized methodologies to explore differences in ictal and interictal spatiotemporal activation patterns based on visual stimuli using fMRI in two patients with unique aura triggers. Both patients underwent separate fMRI sessions during the ictal and interictal periods. The Gaussian Process Classifier (GPC) was used to differentiate these periods by employing a machine learning temporal embedding approach and spatiotemporal activation patterns based on visual stimuli. When restricted to visual and occipital regions, GPC had an improved performance, with accuracy rates for patients A and B of roughly 86–90% and 77–81%, respectively (p < 0.01). The algorithm effectively differentiated visual stimulation and rest periods and identified times when aura symptoms manifested, as evident from the varying predicted probabilities in the GPC models. These findings contribute to our understanding of the role of visual processing and brain activity patterns in migraine with aura and the significance of temporal embedding techniques in examining aura phenomena. This finding has implications for diagnostic tools and therapeutic techniques, especially for patients suffering from aura symptoms.
Graphical Abstract




Similar content being viewed by others
References
Hansen JM, Baca SM, VanValkenburgh P, Charles A (2013) Distinctive anatomical and physiological features of migraine aura revealed by 18 years of recording. Brain 136:3589–3595. https://doi.org/10.1093/brain/awt309
Viana M, Tronvik EA, Do TP et al (2019) Clinical features of visual migraine aura: a systematic review. J Headache Pain 20:64. https://doi.org/10.1186/s10194-019-1008-x
(2018) Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition. Cephalalgia : an Intl J Headache 38:1–211. https://doi.org/10.1177/0333102417738202
Lipton RB, Scher AI, Kolodner K et al (2002) Migraine in the United States: epidemiology and patterns of health care use. Neurology 58:885–894. https://doi.org/10.1212/WNL.58.6.885
Viana M, Sances G, Linde M et al (2017) Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia: an Intl J Headache 37:979–989. https://doi.org/10.1177/0333102416657147
Hadjikhani N, Sanchez del Rio M, Wu O et al (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci 98:4687–4692. https://doi.org/10.1073/pnas.071582498
Hougaard A, Amin FM, Amin F et al (2013) Provocation of migraine with aura using natural trigger factors. Neurology 80:428–431. https://doi.org/10.1212/WNL.0b013e31827f0f10
Lauritzen M (1994) Pathophysiology of the migraine aura. The spreading depression theory. Brain: a J Neurol 117(Pt 1):199–210. https://doi.org/10.1093/brain/117.1.199
Messina R, Filippi M, Goadsby PJ (2018) Recent advances in headache neuroimaging. Curr Opin Neurol 31:379–385. https://doi.org/10.1097/WCO.0000000000000573
Tu Y, Zeng F, Lan L et al (2020) An fMRI-based neural marker for migraine without aura. Neurology 94:e741–e751. https://doi.org/10.1212/wnl.0000000000008962
Messina R, Cetta I, Colombo B, Filippi M (2022) Tracking the evolution of non-headache symptoms through the migraine attack. J Headache Pain 23:149. https://doi.org/10.1186/s10194-022-01525-6
Chong CD, Gaw N, Fu Y et al (2017) Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia 37:828–844. https://doi.org/10.1177/0333102416652091
Jorge-Hernandez F, Chimeno YG, Garcia-Zapirain B et al (2014) Graph theory for feature extraction and classification: a migraine pathology case study. Bio-Med Mater Eng 24:2979–2986. https://doi.org/10.3233/BME-141118
Rocca MA, Harrer JU, Filippi M (2020) Are machine learning approaches the future to study patients with migraine? Neurology 94:291–292. https://doi.org/10.1212/WNL.0000000000008956
Yang H, Zhang J, Liu Q, Wang Y (2018) Multimodal MRI-based classification of migraine: using deep learning convolutional neural network. Biomed Eng Online 17:1–14. https://doi.org/10.1186/s12938-018-0587-0
Fu T, Liu L, Huang X et al (2022) Cerebral blood flow alterations in migraine patients with and without aura: an arterial spin labeling study. J Headache Pain 23:131. https://doi.org/10.1186/s10194-022-01501-0
Shawe-Taylor J, Cristianini N (2004) Kernel methods for pattern analysis. Cambridge University Press
Mitrović K, Petrušić I, Radojičić A et al (2023) Migraine with aura detection and subtype classification using machine learning algorithms and morphometric magnetic resonance imaging data. Front Neurol 14:1106612. https://doi.org/10.3389/fneur.2023.1106612
Gou C, Yang S, Hou Q et al (2023) Functional connectivity of the language area in migraine: a preliminary classification model. BMC Neurol 23:142. https://doi.org/10.1186/s12883-023-03183-w
Dumkrieger G, Chong CD, Ross K et al (2023) The value of brain MRI functional connectivity data in a machine learning classifier for distinguishing migraine from persistent post-traumatic headache. Front Pain Res 3:1012831. https://doi.org/10.3389/fpain.2022.1012831
Hong J, Sun J, Zhang L et al (2022) Neurological mechanism and treatment effects prediction of acupuncture on migraine without aura: study protocol for a randomized controlled trial. Front Neurol 13:981752. https://doi.org/10.3389/fneur.2022.981752
Wei H-L, Xu C-H, Wang J-J et al (2022) Disrupted functional connectivity of the amygdala predicts the efficacy of non-steroidal anti-inflammatory drugs in migraineurs without aura. Front Mol Neurosci 15:819507. https://doi.org/10.3389/fnmol.2022.819507
Cheng S, Zhang X, Zheng H et al (2022) Efficacy prediction of acupuncture treatment for migraine without aura based on multimodal MRI: A study protocol. Front Neurol 13:953921. https://doi.org/10.3389/fneur.2022.953921
Lee CH, Park H, Lee MJ, Park B (2023) Whole-brain functional gradients reveal cortical and subcortical alterations in patients with episodic migraine. Hum Brain Mapp 44:2224–2233. https://doi.org/10.1002/hbm.26204
Mu J, Chen T, Quan S et al (2020) Neuroimaging features of whole-brain functional connectivity predict attack frequency of migraine. Hum Brain Mapp 41:984–993. https://doi.org/10.1002/hbm.24854
Mitchell TM, Hutchinson R, Niculescu RS et al (2004) Learning to decode cognitive states from brain images. Mach Learn 57:145–175. https://doi.org/10.1023/B:MACH.0000035475.85309.1b
Mourão-Miranda J, Friston KJ, Brammer M (2007) Dynamic discrimination analysis: a spatial-temporal SVM. Neuroimage 36:88–99. https://doi.org/10.1016/j.neuroimage.2007.02.020
Chu C, Mourão-Miranda J, Chiu YC et al (2011) Utilizing temporal information in fMRI decoding: classifier using kernel regression methods. Neuroimage 58:560–571. https://doi.org/10.1016/j.neuroimage.2011.06.053
Guidotti R, Del Gratta C, Baldassarre A et al (2015) Visual learning induces changes in resting-state fMRI multivariate pattern of information. J Neurosci : official J Soc Neurosci 35:9786–9798. https://doi.org/10.1523/JNEUROSCI.3920-14.2015
Janoos F, Machiraju R, Singh S, Morocz IÁ (2011) Spatio-temporal models of mental processes from fMRI. Neuroimage 57:362–377. https://doi.org/10.1016/j.neuroimage.2011.03.047
Venkatesh M, Jaja J, Pessoa L (2019) Brain dynamics and temporal trajectories during task and naturalistic processing. Neuroimage 186:410–423. https://doi.org/10.1016/j.neuroimage.2018.11.016
Hagenbeek RE, Rombouts SARB, Van Dijk BW, Barkhof F (2002) Determination of individual stimulus-response curves in the visual cortex. Hum Brain Mapp 17:244–250. https://doi.org/10.1002/hbm.10067
Evans ACC, Collins DLL, Mills SRR, Brown ED, Kelly RL, Peters TM (1993) 3D statistical neuroanatomical models from 305 MRI volumes. In: 1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference, San Francisco, CA, USA. IEEE 3:1813–1817. https://doi.org/10.1109/NSSMIC.1993.373602
Rasmussen CE, Williams CKI (2006) Gaussian processes for machine learning. The MIT Press. Gambridge, Massachusetts
Schrouff J, Rosa MJ, Rondina JM et al (2013) PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics 11:319–337. https://doi.org/10.1007/s12021-013-9178-1
Burges CJ (1998) A tutorial on support vector machines for pattern recognition. Data Min Knowl Disc 2:121–167. https://doi.org/10.1023/A:1009715923555
SB Eickhoff, KE Stephan, H Mohlberg, et al (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data 25 1325-1335 https://doi.org/10.1016/j.neuroimage.2004.12.034
Amunts K, Malikovic A, Mohlberg H et al (2000) Brodmann’s areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage 11:66–84. https://doi.org/10.1006/nimg.1999.0516
Rottschy C, Eickhoff SB, Schleicher A et al (2007) Ventral visual cortex in humans: cytoarchitectonic map** of two extrastriate areas. Hum Brain Mapp 28:1045–1059. https://doi.org/10.1002/hbm.20348
Kujovic M, Zilles K, Malikovic A et al (2013) Cytoarchitectonic map** of the human dorsal extrastriate cortex. Brain Struct Funct 218:157–172. https://doi.org/10.1007/s00429-012-0390-9
Malikovic A, Amunts K, Schleicher A et al (2006) Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex 17:562–574. https://doi.org/10.1093/cercor/bhj181
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239. https://doi.org/10.1016/S1053-8119(03)00169-1
Portugal LCL, Rosa MJ, Rao A et al (2016) Can emotional and behavioral dysregulation in youth be decoded from functional neuroimaging? PLoS ONE 11:e0117603. https://doi.org/10.1371/journal.pone.0117603
Schrouff J, Monteiro JM, Portugal L et al (2018) Embedding anatomical or functional knowledge in whole-brain multiple kernel learning models. Neuroinformatics 16:117–143. https://doi.org/10.1007/s12021-017-9347-8
Cao Y, Aurora SK, Nagesh V et al (2002) Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology 59:72–78. https://doi.org/10.1212/wnl.59.1.72
Hougaard A, Amin FM, Hoffmann MB et al (2014) Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp 35:2714–2723. https://doi.org/10.1002/hbm.22361
Silvestro M, Tessitore A, Di Nardo F, et al (2021) Functional connectivity changes in complex migraine aura: beyond the visual network. Euro J Neurol 15061. https://doi.org/10.1111/ene.15061
Tedeschi G, Russo A, Conte F et al (2016) Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia an Intl J Headache 36:139–147. https://doi.org/10.1177/0333102415584360
Tessitore A, Russo A, Conte F et al (2015) Abnormal connectivity within executive resting-state network in migraine with aura. Headache 55:794–805. https://doi.org/10.1111/head.12587
Zhang D, Huang X, Su W, et al (2020) Altered lateral geniculate nucleus functional connectivity in migraine without aura: a resting-state functional MRI study. J Headache Pain 21 https://doi.org/10.1186/s10194-020-01086-6
Arngrim N, Hougaard A, Ahmadi K et al (2017) Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Ann Neurol 82:925–939. https://doi.org/10.1002/ana.25096
Rasmussen AH, Kogelman LJA, Kristensen DM et al (2020) Functional gene networks reveal distinct mechanisms segregating in migraine families. Brain: J Neurol 143:2945–2956. https://doi.org/10.1093/brain/awaa242
Russell MB, Olesen J (1996) A nosographic analysis of the migraine aura in a general population. Brain: J Neurol 119(Pt 2):355–361. https://doi.org/10.1093/brain/119.2.355
Marquand AF, De Simoni S, O’Daly OG et al (2011) Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacol 36:1237–1247. https://doi.org/10.1038/npp.2011.9
Portugal LCL, Ramos TC, Fernandes O, et al (2023) Machine learning applied to fMRI patterns of brain activation in response to mutilation pictures predicts PTSD symptoms. https://doi.org/10.21203/rs.3.rs-2928305/v1
Mourão-Miranda J, Oliveira L, Ladouceur CD et al (2012) Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS ONE 7:e29482. https://doi.org/10.1371/journal.pone.0029482
Marquand A, Howard M, Brammer M et al (2010) Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage 49:2178–2189. https://doi.org/10.1016/j.neuroimage.2009.10.072
Varoquaux G, Raamana PR, Engemann DA et al (2017) Assessing and tuning brain decoders: cross-validation, caveats, and guidelines. Neuroimage 145:166–179. https://doi.org/10.1016/j.neuroimage.2016.10.038
Gill S, Mouches P, Hu S et al (2020) Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheim Dis 75:277–288. https://doi.org/10.3233/jad-191169
Acknowledgements
The authors would like to express their gratitude to all the subjects who participated in the study; to Professor Maurice Vincent for important discussions; to Luke Barbara for English revision of the manuscript; and Danielle Pimentel and Tania Maria Netto for technical support. We express our acknowledgements to “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for their support.
Funding
OFJ, MA, and LRR were previously funded by CAPES foundation—“Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”—Brazil. OFJ is funded by FAPERJ—“Fundação de Aparo à Pesquisa do Estado do Rio de Janeiro”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Federal University of Rio de Janeiro, Brazil, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethical approval can be found in Brazilian’s National Health Council webpage at: https://plataformabrasil.saude.gov.br (identifier #429.485/2013). Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernandes, O., Ramos, L.R., Acchar, M.C. et al. Migraine aura discrimination using machine learning: an fMRI study during ictal and interictal periods. Med Biol Eng Comput (2024). https://doi.org/10.1007/s11517-024-03080-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11517-024-03080-5