Abstract
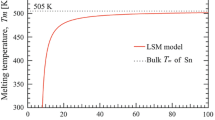
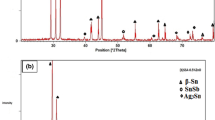
The commercial market of Sn–Pb solder is gradually decreasing due to its toxicity, calling for Pb-free substitute materials. Sn–Ag alloy is a potential candidate in terms of good mechanical property. The major problematic issue of using Sn–Ag is their high melting temperature, consequently this study is dedicated to lowering the melting temperature of Sn3.5Ag (wt%) alloy by develo** nanomaterials using a chemical reduction approach. The resultant nanocrystalline Sn3.5Ag is characterized by field emission scanning electron microscope. The size dependence of the melting temperature is discussed based on differential scanning calorimetry results. We have reduced the melting temperature to 209.8 °C in the nanocrystalline Sn3.5Ag of (32.4 ± 8.0) nm, compared to ~221 °C of the bulk alloy. The results are consistent with the prediction made by a relevant theoretical model, and it is possible to further lower the melting temperature using the chemical reduction approach developed by this study.



Similar content being viewed by others
References
Shen J, Liu Y, Gao H (2006) Abnormal growth of Ag3Sn intermetallic compounds in Sn–Ag lead-free solder. Chin Sci Bull 51:1766–1770
Zhang X, Yuan Z, Zhao H et al (2010) Wetting behavior and interfacial characteristic of Sn–Ag–Cu solder alloy on Cu substrate. Chin Sci Bull 55:797–801
Cho MG, Kim HY, Seo SK et al (2009) Enhancement of heterogeneous nucleation of β-Sn phases in Sn-rich solders by adding minor alloying elements with hexagonal closed packed structures. Appl Phys Lett 95:021905
Keller J, Baither D, Wilke U et al (2011) Mechanical properties of Pb-free SnAg solder joints. Acta Mater 59:2731–2741
Zhang L, Han JG, Guo YH et al (2014) Effect of rare earth Ce on the fatigue life of SnAgCu solder joints in WLCSP device using fem and experiments. Mater Sci Eng A 597:219–224
Lai SL, Guo JY, Petrova V et al (1996) Size-dependent melting properties of small tin particles: nanocalorimetric measurements. Phys Rev Lett 77:99–102
Suryanarayana C, Ivanov E, Boldyrev VV (2001) The science and technology of mechanical alloying. Mater Sci Eng A 304–306:151–158
Zhang H, Tang WM, Xu GQ et al (2010) Synthesis of Sn–Ag binary alloy powders by mechanical alloying. Mater Chem Phys 122:64–68
Birringer R, Gleiter H, Klein HP et al (1984) Nanocrystalline materials an approach to a novel solid structure with gas-like disorder. Phys Lett A 102:365–369
Zhao B, Li L, Yang B et al (2013) Structure observation of single solidified droplet by in situ controllable quenching based on nanocalorimetry. J Alloy Compd 580:386–391
Gao YL, Zou CD, Yang B et al (2009) Nanoparticles of SnAgCu lead-free solder alloy with an equivalent melting temperature of SnPb solder alloy. J Alloy Compd 484:777–781
Raveendran P, Fu J, Wallen SL (2003) Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc 125:13940–13941
Jiang H, Moon K, Hua F et al (2007) Synthesis and thermal and wetting properties of tin/silver alloy nanoparticles for low melting point lead-free solders. Chem Mater 19:4482–4485
Hsiao LY, Duh JG (2005) Synthesis and characterization of lead-free solders with Sn–3.5Ag–xCu (x = 0.2, 0.5, 1.0) alloy nanoparticles by the chemical reduction method. J Electrochem Soc 152:105–109
Zhang W, Zou C, Zhao B et al (2014) Size control and its mechanism of SnAg nanoparticles. Trans Nonferr Met Soc 24:750–757
Mei QS, Lu K (2007) Melting and superheating of crystalline solids: from bulk to nanocrystals. Prog Mater Sci 52:1175–1262
Sun J, Simon SL (2007) The melting behavior of aluminum nanoparticles. Thermochim Acta 463:32–40
Campbell CT, Parker SC, Starr DE (2002) The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 298:811–814
Pan D, Liu LM, Slater B et al (2011) Melting the ice: on the relation between melting temperature and size for nanoscale ice crystals. ACS Nano 5:4562–4569
Abtew M, Selvaduray G (2000) Lead-free solders in microelectronics. Mater Sci Eng, R 27:95–141
Guan W, Verma SC, Gao Y et al (2006) Characterization of nanoparticles of lead free solder alloys. In: 1st electronics system integration technology conference, Institute of Electrical and Electronics Engineers, Dresden, 5–7 Sept 2006
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50971086, 51171105).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhao, B., Zhang, W., Zou, C. et al. Low melting point nanocrystalline Sn–Ag solder synthesized by a refined chemical reduction method. Chin. Sci. Bull. 59, 4147–4151 (2014). https://doi.org/10.1007/s11434-014-0528-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-014-0528-7