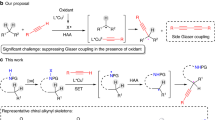
Abstract
An efficient catalytic asymmetric dearomatizing amination of 2-naphthols and phenols catalyzed by N,N′-dioxide-copper(I) complex as a chiral catalyst was presented. A variety of optically active β-naphthalenone compounds with a nitrogen-containing quaternary carbon stereocenter were obtained with high yield and enantioselectivity under mild reaction conditions. Mechanistic studies indicated that this Csp2–N dearomatizing coupling proceeds via 1,3-reductive elimination of phenolate-CuIII-amino intermediate in five-membered ring transition states. The origin of enantioselectivity has also been elucidated based on density functional theory calculations.
Similar content being viewed by others
References
Randolph JT, Flentge CA, Huang PP, Hutchinson DK, Klein LL, Lim HB, Mondal R, Reisch T, Montgomery DA, Jiang WW, Masse SV, Hernandez LE, Henry RF, Liu Y, Koev G, Kati WM, Stewart KD, Beno DWA, Molla A, Kempf DJ. J Med Chem, 2009, 52: 3174–3183
Wu WT, Zhang L, You SL. Chem Soc Rev, 2016, 45: 1570–1580
**g C, Farndon JJ, Bower JF. Chem Record, 2021, 21: 2909–2926
Sarkar D, Ghosh MK, Rout N, Kuila P. New J Chem, 2017, 41: 3715–3718
Scheffler G, Seike H, Sorensen EJ. Angew Chem Int Ed, 2000, 39: 4593–4596
Canesi S, Bouchu D, Ciufolini MA. Org Lett, 2005, 7: 175–177
Jain N, Ciufolini M. Synlett, 2015, 26: 631–634
Liang H, Ciufolini MA. Org Lett, 2010, 12: 1760–1763
Yi JC, Tu HF, You SL. Org Biomol Chem, 2018, 16: 8700–8703
Wardrop DJ, Burge MS. J Org Chem, 2005, 70: 10271–10284
**a ZL, Zheng C, Xu RQ, You SL. Nat Commun, 2019, 10: 3150
Wang SG, Yin Q, Zhuo CX, You SL. Angew Chem, 2015, 127: 657–660
Lian X, Lin L, Wang G, Liu X, Feng X. Chem Eur J, 2015, 21: 17453–17458
Chen Y, Jia SK, **ao X, Wang MC, Huang L, Mei GJ. Org Lett, 2023, 25: 4740–4744
Maji U, Mondal BD, Guin J. Org Lett, 2023, 25: 2323–2327
Hwang Y, Park Y, Kim YB, Kim D, Chang S. Angew Chem Int Ed, 2018, 57: 13565–13569
Yu JS, Espinosa M, Noda H, Shibasaki M. J Am Chem Soc, 2019, 141: 10530–10537
Yi J, Wu Z, You S. Eur J Org Chem, 2019, 2019(33): 5736–5739
Tanaka K, Mori Y, Narasaka K. Chem Lett, 2004, 33: 26–27
Ma XF, Farndon JJ, Young TA, Fey N, Bower JF. Angew. Chem. Int. Ed., 2017, 56: 14531–14535
Farndon JJ, Ma X, Bower JF. J Am Chem Soc, 2017, 139: 14005–14008
Jia L, Gao S, **e J, Luo M. Adv Synth Catal, 2016, 358: 3840–3846
Gu QS, Li ZL, Liu XY. Acc Chem Res, 2020, 53: 170–181
Kainz QM, Matier CD, Bartoszewicz A, Zultanski SL, Peters JC, Fu GC. Science, 2016, 351: 681–684
Chen C, Peters JC, Fu GC. Nature, 2021, 596: 250–256
Cho H, Suematsu H, Oyala PH, Peters JC, Fu GC. J Am Chem Soc, 2022, 144: 4550–4558
Chen JJ, Fang JH, Du XY, Zhang JY, Bian JQ, Wang FL, Luan C, Liu WL, Liu JR, Dong XY, Li ZL, Gu QS, Dong Z, Liu XY. Nature, 2023, 618: 294–300
Chen JJ, Zhang JY, Fang JH, Du XY, **a HD, Cheng B, Li N, Yu ZL, Bian JQ, Wang FL, Zheng JJ, Liu WL, Gu QS, Li ZL, Liu XY. J Am Chem Soc, 2023, 145: 14686–14696
Zhang YF, Dong XY, Cheng JT, Yang NY, Wang LL, Wang FL, Luan C, Liu J, Li ZL, Gu QS, Liu XY. J Am Chem Soc, 2021, 143: 15413–15419
Zhang YF, Wang JH, Yang NY, Chen Z, Wang LL, Gu QS, Li ZL, Liu XY. Angew Chem Int Ed, 2023, 62: e202302983
Huang C, Wan Z, Zhu A, Chen C. Chin J Chem, 2024, 42: 1161–1174
Dai L, Chen Y, **ao L, Zhou Q. Angew Chem Int Ed, 2023, 62: e202304427
Cheng X, Chang X, Yang Y, Zhang Z, Li J, Li Y, Zhao W, Chung LW, Teng H, Dong XQ, Wang CJ. Sci China Chem, 2023, 66: 3193–3204
Yao ZL, Wang L, Shao NQ, Guo YL, Wang DH. ACS Catal, 2019, 9: 7343–7349
Gao Y, Li H, Yang S, Huo Y, Chen Q, Li X, Wang Z, Hu XQ. Sci China Chem, 2024, 67: 595–603
Bai L, Luo X, Ge Y, Wang H, Liu J, Wang Y, Luan X. CCS Chem, 2022, 4: 1054–1064
Zhao X, Liu X, Mei H, Guo J, Lin L, Feng X. Angew Chem Int Ed, 2015, 54: 4032–4035
Wang L, Zhou Y, Su Z, Zhang F, Cao W, Liu X, Feng X. Angew Chem Int Ed, 2022, 61: e202211785
Zhang Y, Liao Y, Liu X, Xu X, Lin L, Feng X. Chem Sci, 2017, 8: 6645–6649
Ge S, Kang T, Lin L, Zhang X, Zhao P, Liu X, Feng X. Chem Commun, 2017, 53: 11759–11762
Liu W, Pu M, He J, Zhang T, Dong S, Liu X, Wu YD, Feng X. J Am Chem Soc, 2021, 143: 11856–11863
He Q, Pu MP, Jiang Z, Wang H, Feng X, Liu X. J Am Chem Soc, 2023, 145: 15611–15618
Xu N, Pu M, Yu H, Yang G, Liu X, Feng X. Angew Chem Int Ed, 2024, 63: e202314256
Liu XH, Lin LL, Feng XM. Acc Chem Res, 2011, 44: 574–587
Liu X, Lin L, Feng X. Org Chem Front, 2014, 1: 298
Liu X, Zheng H, **a Y, Lin L, Feng X. Acc Chem Res, 2017, 50: 2621–2631
Liu X, Dong S, Lin L, Feng X. Chin J Chem, 2018, 36: 791–797
Cao W, Liu X, Feng X. Chin Sci Bull, 2020, 65: 2941–2951
Wang MY, Li W. Chin J Chem, 2021, 39: 969–984
Dong S, Liu X, Feng X. Acc Chem Res, 2022, 55: 415–428
Chen DF, Gong LZ. Org Chem Front, 2023, 10: 3676–3683
He YM, Cheng YZ, Duan Y, Zhang YD, Fan QH, You SL, Luo S, Zhu SF, Fu XF, Zhou QL. CCS Chem, 2023, 5: 2685–2716
Li J, Ning L, Tan Q, Feng X, Liu X. Org Chem Front, 2022, 9: 6312–6318
Deposition Number 2271770 (3an) and 2256161 (3ap) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Zhu X, Li Y, Bao H. Chin J Chem, 2023, 41: 3097–3114
Dong S, Cao W, Pu M, Liu X, Feng X. CCS Chem, 2023, 5: 2717–2735
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22188101) and Sichuan Science and Technology Program (2021YJ0561). We appreciate Dr. Bo Gao from the Analytical & Testing Center of Sichuan University for help with LC-MS characterization. We thank Dr. Yuqiao Zhou (Sichuan University) for assistance in X-ray analysis and Dr. Hanjiao Chen (Sichuan University) for assistance with EPR measurement and simulation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://springer.longhoe.net/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Yihuo, A., Pu, M., Tan, Z. et al. Copper-catalyzed asymmetric dearomatizing amination of 2-naphthols: Csp2–N coupling via 1,3-reductive elimination. Sci. China Chem. (2024). https://doi.org/10.1007/s11426-024-2030-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11426-024-2030-3