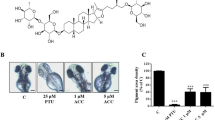
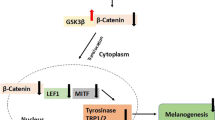
Abstract
Abnormal melanin synthesis causes hyperpigmentation disorders, such as chloasma, freckles, and melanoma, which are highly multiple and prevalent. There were few reports on the anti-melanogenic effect of Curcuma wenyu** Y.H. Chen et C. Ling, and the bioactive compound has not been elucidated as well. The study aims to investigate the anti-melanogenic effect of C. wenyu**, and identify the bioactive compound, and further explore its underlying mechanism. Our results showed that the Petroleum ether fraction extracted from C. wenyu** rhizome had a significant anti-melanogenic effect, and germacrone isolated from it was confirmed as the major bioactive compound. To our data, germacrone significantly inhibited tyrosinase (TYR) activity, reduced melanosome synthesis, reduced dendrites formation of B16F10 cells, and melanosome transport to keratinocytes. Moreover, germacrone effectively decreased the hyperpigmentation in zebrafish and the skin of guinea pigs in vivo. Western-blot analysis showed that germacrone down-regulated the expression of TYR, TRP-1, TRP-2, Rab27a, Cdc42, and MITF proteins via the activation of the MAPK signaling pathway. Taken together, germacrone is an effective bioactive compound for melanogenesis inhibition. Our studies suggest that germacrone may be considered a potential candidate for skin whitening.
Graphical abstract







Similar content being viewed by others
Abbreviations
- TYR:
-
Tyrosinase
- TRP-1:
-
Tyrosinase-relative protein 1
- TRP-2:
-
Tyrosinase-relative protein 2
- MITF:
-
Microphthalmia-associated transcription factor
- L-DOPA:
-
3,4-Dihydroxyphenylalanine
- IBMX:
-
3-Isobutyl-1-methylxanthine
- PTU:
-
N-phenylthiourea
- MAPKs:
-
Mitogen-activated protein kinase
- TEM:
-
Transmission electron microscope
- HPLC:
-
High-performance liquid chromatography
- Cdc42:
-
Cell division cycle protein 42
- PMEL-17:
-
Pre-melanosome protein 17
- Rab27a:
-
Ras-related protein Rab27a
References
Pavan WJ, Sturm RA (2019) The genetics of human skin and hair pigmentation. Annu Rev Genomics Hum Genet 20:41–72. https://doi.org/10.1146/annurev-genom-083118-015230
An XH, Lv JP, Wang FF (2022) Pterostilbene inhibits melanogenesis, melanocyte dendricity and melanosome transport through cAMP/PKA/CREB pathway. Eur J Pharmacol 932:175231. https://doi.org/10.1016/j.ejphar.2022.175231
Tang H, Yang L, Wu L et al (2021) Kaempferol, the melanogenic component of sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J Pharmacol Sci 147(4):348–357. https://doi.org/10.1016/j.jphs.2021.08.009
Jeong HS, Gu GE, Jo AR et al (2015) Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase. Eur J Pharmacol 761:19–27. https://doi.org/10.1016/j.ejphar.2015.04.028
Liu F, Qu LK, Li H et al (2022) Advances in biomedical fFunctions of natural whitening substances in the treatment of skin pigmentation diseases. Pharmaceutics 14(11):2308. https://doi.org/10.3390/pharmaceutics14112308
D’Mello S, Finlay GJ, Baguley BC, Askarian-Amiri ME (2016) Signaling pathways in melanogenesis. Int J Mol Sci 17(7):1144. https://doi.org/10.3390/ijms17071144
Hong C, Yang LL, Zhang YF, Li YM, Wu HL (2022) Epimedium brevicornum Maxim. extract exhibits pigmentation by melanin biosynthesis and melanosome biogenesis transfer. Front Pharmacol 13:963160. https://doi.org/10.3389/fphar.2022.963160
Tu CX, Lin M, Lu SS, Qi XY, Zhang RX, Zhang YY (2012) Curcumin inhibits melanogenesis in human melanocytes. Phytother Res 26(2):174–179. https://doi.org/10.1002/ptr.3517
Lv JP, Yang Y, Jia BY, Li SQ, Zhang XM, Gao RY (2021) The inhibitory effect of curcumin derivative J147 on melanogenesis and melanosome transport by facilitating ERK-mediated MITF degradation. Front Pharmacol 12:783730. https://doi.org/10.3389/fphar.2021.783730
Zhou SH, Zeng HL, Huang JH et al (2021) Epigenetic regulation of melanogenesis. Ageing Res Rev 69:101349. https://doi.org/10.1016/j.arr.2021.101349
Liu JZ, Jiang R, Zhou JY et al (2021) Salicylic acid in ginseng root alleviates skin hyperpigmentation disorders by inhibiting melanogenesis and melanosome transport. Eur J Pharmacol 910:174498. https://doi.org/10.1016/j.ejphar.2021.174458
Ishida M, Arai SP, Ohbayashi N, Fukuda M (2014) The GTPase-deficient Rab 27A(Q78L) mutant inhibits melanosome transport in melanocytes through trap** of Rab27A effector protein Slac2-a/melanophilin in their cytosol. J Biol Chem 289(16):11059–11067. https://doi.org/10.1074/jbc.M114.552281
Wang YP, Li Z, Wu W et al (2022) TRPA1 promotes melanosome phagocytosis in keratinocytes via PAR-2/ CYLD axis. J Dermatol Sci 106(3):181–188. https://doi.org/10.1016/j.jdermsci.2022.05.005
Li YH, Wu YC, Li YM, Guo FJ (2021) Review of the traditional uses, phytochemistry, and pharmacology of Curcuma wenyu** YH Chen et C Ling. J Ethnopharmacol 269:113689. https://doi.org/10.1016/j.jep.2020.113689
Li YH, Wang H, Wang H, Wu YC, Li YM, Guo FJ (2022) Nine new sesquiterpenes from Curcuma wenyu** rhizomes. Fitoterapia 158:105167. https://doi.org/10.1016/j.fitote.2022.105167
Chen LJ, Liu JW, Wang H, Li YH, Li YM, Guo FJ (2022) Four new sesquiterpenes from Curcuma wenyu**. Fitoterapia 163:105344. https://doi.org/10.1016/j.fitote.2022.105344
Liu R, Pei Q, Shou T, Zhang WJ, Hu JLW (2019) Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyu** extract against human renal cell carcinoma A498 cells. Int J Nanomedicine 14:4091–4103. https://doi.org/10.2147/IJN.S203222
Gao SY, **a GY, Wang LQ et al (2017) Sesquiterpenes from Curcuma wenyu** with their inhibitory activities on nitric oxide production in RAW 264. 7 cells. Nat Prod Res 31(5):548–554. https://doi.org/10.1080/14786419.2016.1205053
**a Q, Wang X, Xu DJ, Chen XH, Chen FH (2012) Inhibition of platelet aggregation by curdione from Curcuma wenyu** essential Oil. Thromb Res 130(3):409–414. https://doi.org/10.1016/j.thromres.2012.04.005
**e H, Su D, Zhang J et al (2020) Raw and vinegar processed Curcuma wenyu** regulates hepatic fibrosis via bloking TGF-β/Smad signaling pathways and up-regulation of MMP-2/TIMP-1 ratio. J Ethnopharmacol 246:111768. https://doi.org/10.1016/j.jep.2019.01.045
Kwon PK, Kim SW, De R, Jeong SW, Kim KT (2021) Isoprocurcumenol supports keratinocyte growth and survival through epidermal growth factor receptor activation. Int J Mol Sci 22(22):12579. https://doi.org/10.3390/ijms222212579
Wang H, Li XY, Li YH, Wu HL, Li YM, Guo FJ (2022) Four new sesquiterpenes from the rhizomes of Curcuma wenyu**. Phytochem Lett 52:143–148. https://doi.org/10.1016/j.phytol.2022.11.002
Rothberg BEG, Moeder CB, Kluger H et al (2008) Nuclear to non-nuclear Pmel17/gp100 expression (HMB45 staining) as a discriminator between benign and malignant melanocytic lesions. Mod Pathol 21(9):1121–1129. https://doi.org/10.1038/modpathol.2008.100
Liu L, Zhong M, Dong J, Chen MJ, Shang J, Yue YY (2020) 5-Hydroxytryptamine (5-HT) positively regulates pigmentation via inducing melanoblast specification and melanin synthesis in Zebrafish Embryos. Biomolecules 10(9):1344. https://doi.org/10.3390/biom10091344
Choi HY, Yoon JH, Youn KJ, Jun M (2022) Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed Pharmacother 147:112651. https://doi.org/10.1016/j.biopha.2022.112651
Wang HM, Qu LQ, Ng PLJ et al (2022) Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine 98:153941. https://doi.org/10.1016/j.phymed.2022.153941
Feng D, Fang ZX, Zhang PZ (2022) The melanin inhibitory effect of plants and phytochemicals: a systematic review. Phytomedicine 107:154449. https://doi.org/10.1016/j.phymed.2022.154449
Wang ZR, Zhuo F, Chu PG, Yang XL, Zhao G (2019) Germacrone alleviates collagen-induced arthritis via regulating Th1/ Th2 balance and NF-kB activation. Biochem Biophys Res Commun 518(3):560–564. https://doi.org/10.1016/j.bbrc.2019.08.084
Liao QJ, Qian ZX, Liu R, An LW, Chen XL (2013) Germacrone inhibits early stages of influenza virus infection. Antiviral Res 100(3):578–588. https://doi.org/10.1016/j.antiviral.2013.09.021
Zhao Y, Cai J, Shi KH et al (2021) Germacrone induces lung cancer cell apoptosis and cell cycle arrest via the Akt/MDM2/p53 signaling pathway. Mol Med Rep 23(6):452. https://doi.org/10.3892/mmr.2021.12091
Ji D, Zhao Q, Qin YW et al (2021) Germacrone improves liver fibrosis by regulating the PI3K/AKT/mTOR signalling pathway. Cell Biol Int 45(9):1866–1875. https://doi.org/10.1002/cbin.11607
Zhuang SJ, Liu BG, Guo SF et al (2021) Germacrone alleviates neurological deficits following traumatic brain injury by modulating neuroinflammation and oxidative stress. BMC Complement Med Ther 21(1):6. https://doi.org/10.1186/s12906-020-03175-0
Hashim FJ, Vichitphan S, Han JH, Vichitphan K (2021) Alternative approach for specific tyrosinase inhibitor screening: uncompetitive inhibition of tyrosinase by Moringa oleifera. Molecules 26(15):4576. https://doi.org/10.3390/molecules26154576
Li J, Feng L, Liu L et al (2021) Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur J Med Chem 224:113744. https://doi.org/10.1016/j.ejmech.2021.113744
Hu SH, Huang JH, Pei SY et al (2019) Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J Cell Physiol 234(5):7330–7340. https://doi.org/10.1002/jcp.27492
Pillaiyara T, Manickamb M, Jung SH (2017) Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal 40:99–115. https://doi.org/10.1016/j.cellsig.2017.09.004
Karunarathne WAHM, Molagoda IMN, Kim MS et al (2019) Flumequine-mediated upregulation of p38 MAPK and JNK results in melanogenesis in B16F10 cells and Zebrafish Larvae. Biomolecules 9(10):596. https://doi.org/10.3390/biom9100596
Jung ES, Kim JH, Kim MO et al (2016) Afzelin positively regulates melanogenesis through the p38 MAPK pathway. Chem Biol Interact 254:167–172. https://doi.org/10.1016/j.cbi.2016.06.010
Yamaguchi YJ, Hearing VJ (2009) Physiological factors that regulate skin pigmentation. BioFactors 35(2):193–199. https://doi.org/10.1002/biof.29
Lv JP, An XH, Jiang SZ, Yang Y, Song GQ, Gao RY (2020) Protoporphyrin IX stimulates melanogenesis, melanocyte dendricity, and melanosome transport through the cGMP/PKG pathway. Front Pharmacol 11:569368. https://doi.org/10.3389/fphar.2020.569368
Boissy RE (2003) Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol 12(Suppl 2):5–12. https://doi.org/10.1034/j.1600-0625.12.s2.1.x
Bento-Lopes L, Cabaço LC, Charneca J et al (2023) Melanin’s journey from melanocytes to keratinocytes: uncovering the molecular mechanisms of melanin transfer and processing. Int J Mol Sci 24(14):11289. https://doi.org/10.3390/ijms241411289
Choi EJ, Kang TG, Kim J, Hwang JK (2011) Macelignan inhibits melanosome transfer mediated by protease-activated receptor-2 in keratinocytes. Biol Pharm Bull 34(5):748–754. https://doi.org/10.1248/bpb.34.748
Acknowledgements
An appreciated thank to Dr. Yingchun Wu for identifying the plant.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
**aoye Li: Conceptualization, Investigation, Data curation, Writing—original draft. Lijia Chen: Methodology. Hong Wang: Methodology. Yiming Li: Supervision. Huali Wu: Supervision, Review. Fujiang Guo: Supervision, Writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Chen, L., Wang, H. et al. Germacrone, isolated from Curcuma wenyu**, inhibits melanin synthesis through the regulation of the MAPK signaling pathway. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01818-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01818-x