Abstract
Purpose
Nano silicon particles (nSiO2) is one of the most widely used industrial engineered nanomaterials (ENMs). The extensive applications of nSiO2 may pose potential risks to aquatic ecosystems and human health. Humic acid (HA) is a major component of soil and water that exists widely in the natural environment and adsorbs to the surface of nanoparticles, which affects the fate and transport of ENMs in soil. Therefore, it triggers the necessity to study the chemical reaction of HA controlling the sedimentation and transport of nSiO2.
Materials and methods
The sedimentation kinetics and transport breakthrough curves of nSiO2 with/without HA in water-saturated porous media were studied in two electrolyte (NaCl and CaCl2) solutions. The likely mechanisms were explored with both multiple technologies and numerical modeling including TEM-EDX, particle size distribution, zeta potentials, and the two-site kinetic attachment model (TSKAM).
Results and discussion
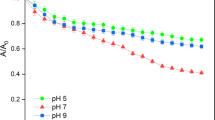
Our experimental results showed that the existence of HA generally increased the suspensivity and the transportability of nSiO2 in NaCl and CaCl2 solutions in packed sand columns at acidic pH. This result was attributed to the HA adsorption leading to the more negatively charged surface and the smaller size of nSiO2 aggregates. However, the formation of coordination complexes associated with larger cluster among nSiO2 between HA and Ca2+ contributed to the increased sedimentation of nSiO2 at alkaline pH. Subsequently, the presence of HA inhibited the transport of nSiO2 in CaCl2 solution at pH 9.0. Comparably, in NaCl at pH 9.0, HA showed the negligible effect on the nSiO2 deposition in sand. Both the attachment and detachment parameters, which were obtained from fitting the breakthrough curves of ENMs using the TSKAM, could be used to well describe the transport behavior of nSiO2 with HA under various conditions. In particular, the irreversible attachment parameters at site 2 on sand were positively related to the retention of nSiO2 with HA.
Conclusions
The fate and transport of nSiO2 can be distinctly affected by HA depending on the ion composition, ion strength, and pH in soil. This study will provide insights for assessing the mobility of nSiO2 with HA in subsurface soil and aquatic environments.






Similar content being viewed by others
References
Adams LK, Lyon DY, Alvarez PJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40(19):3527–3532
Amirbahman A, Olson TM (1995) Deposition kinetics of humic matter-coated hematite in porous media in the presence of Ca2+. Colloids Surf A Physicochem Eng Asp 99(1):1–10
Bayat AE, Junin R, Shamshirband S, Chong WD (2015) Transport and retention of engineered Al2O3, TiO2, and SiO2 nanoparticles through various sedimentary rocks. Sci Rep 5(1):1–12
Ben-Moshe T, Dror I, Berkowitz B (2010) Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 81(3):387–393
Bolster CH, Mills AL, Hornberger GM, Herman JS (1999) Spatial distribution of deposited bacteria following miscible displacement experiments in intact cores. Water Resour Res 35(6):1797–1807
Bradford SA, Simunek J, Bettahar M, van Genuchten MT, Yates SR (2003) Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ Sci Technol 37(10):2242–2250
Bradford SA, Kim HN, Haznedaroglu BZ, Torkzaban S, Walker SL (2009) Coupled factors influencing concentration-dependent colloid transport and retention in saturated porous media. Environ Sci Technol 43(18):6996–7002
Chen G, Liu X, Su C (2012) Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ Sci Technol 46(13):7142–7150
Chen M, Xu N, Cao X, Zhou K, Chen Z (2015) Facilitated transport of anatase titanium dioxides nanoparticles in the presence of phosphate in saturated sands. J Colloid Interface Sci 451:134–143
Chen M, Xu N, Christodoulatos C, Wang D (2018) Synergistic effects of phosphorus and humic acid on the transport of anatase titanium dioxide nanoparticles in water-saturated porous media. Environ Pollut 243:1368–1375
Cho JW, Sul KI (2001) Characterization and properties of hybrid composites prepared from poly (vinylidene fluoride–tetrafluoroethylene) and SiO2. Polymer 42(2):727–736
Cho M, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, Kim HO, Sheen YY, Jeong J (2009) The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol Lett 189:177–183
Chowdhury I, Cwiertny DM, Walker SL (2012) Combined factors influencing the aggregation and deposition of nano-TiO2 in the presence of humic acid and bacteria. Environ Sci Technol 46(13):6968–6976
Cochrane H, Lin CS (1993) The influence of fumed silica properties on the processing, curing, and reinforcement properties of silicone rubber. Rubber Chem Technol 66(1):48–60
Diener L, Wick P, Kaiser JP (2013) Nanoparticles in paints: A new strategy to protect façades and surfaces? J Phys Conf Ser 429:12036–12045
Doshi R, Braida W, Christodoulatos C, Wazne M, Gregory O (2008) Nano-aluminum: transport through sand columns and environmental effects on plants and soil communities. Environ Res 106(3):296–303
Esfahani A, Firouzi A, Sayyad G, Kiasat A (2014) Transport and retention of polymer-stabilized zero-valent iron nanoparticles in saturated porous media: effects of initial particle concentration and ionic strength. J Ind Eng Chem 20(5):2671–2679
Espinasse B, Hotze EM, Wiesner MR (2007) Transport and retention of colloidal aggregates of C60 in porous media: effects of organic macromolecules, ionic composition, and preparation method. Environ Sci Technol 41(21):7396–7402
Fisher-Power L, Cheng T (2018) Nanoscale titanium dioxide (nTiO2) transport in natural sediments: importance of soil organic matter and Fe/Al oxyhydroxides. Environ Sci Technol 52(5):2668–2676
Fruijtier-Polloth C (2012) The toxicological mode of action and the safety of synthetic amorphous silica—a nanostructured material. Toxicology 294(2–3):61–79
Guggenberger G, Rodionov A, Shibistova O, Grabe M, Kasansky OA, Fuchs H, Mikheyeva N, Zrazhevskaya G, Flessa H (2008) Storage and mobility of black carbon in permafrost soils of the forest tundra ecotonein Northern Siberia. Glob Chang Biol 14(6):1367–1381
Hahn MW, O’meliae CR (2004) Deposition and reentrainment of brownian particles in porous media under unfavorable chemical conditions: some concepts and applications. Environ Sci Technol 38(1):210–220
Jiang X, Tong M, Li H, Yang K (2010) Deposition kinetics of zinc oxide nanoparticles on natural organic matter coated silica surfaces. J Colloid Interface Sci 350(2):427–434
Johnson PR, Elimelech M (1995) Dynamics of colloid deposition in porous media: blocking based on random sequential adsorption. Langmuir 11(3):801–812
Johnson RL, Johnsinm GO, Nurmi JT, Tratnyek PG (2009) Natural organic matter enhanced mobility of nano zerovalent iron. Environ Sci Technol 43(14):5455–5460
Jung B, O’Carroll D, Sleep B (2014) The influence of humic acid and clay content on the transport of polymer-coated iron nanoparticles through sand. Sci Total Environ 496:155–164
Kretzschmar R, Borkovec M, Grolimund D, Elimelech M (1999) Mobile subsurface colloids and their role in contaminant transport. Adv Agron 66(08):121–193
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI (2012) Cheminform abstract: mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 41(7):2590–2605
Liang Y, Bradford S, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Retention and remobilization of stabilized silver nanoparticles in an undisturbed loamy sand soil. Environ Sci Technol 47(21):12229–12237
Liu X, Wazne M, Chou T, **ao R, Xu S (2011) Influence of Ca2+ and Suwannee River Humic Acid on aggregation of silicon nanoparticles in aqueous media. Water Res 45(1):105–112
Liu C, Xu N, Feng G, Zhou D, Cheng X, Li Z (2017) Hydrochars and phosphate enhancing the transport of nanoparticle silica in saturated sands. Chemosphere 189:213–223
Loon CK, Menachem E (2008) Interaction of fullerene (C60) nanoparticles with humic acid and alginate coated silica surfaces: measurements, mechanisms, and environmental implications. Environl Sci Technol 42(20):7607–7614
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7:39–70
Nel A, **a T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627
Oberdörster G, Ferin J, Lehnert BE (1994) Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102(suppl 5):173–179
Redman JA, Grant SB, Olson TM, Estes MK (2001) Pathogen filtration, heterogeneity, and the potable reuse of wastewater. Environ Sci Technol 35(9):1798–1805
Redman AD, Macalady DL, Ahmann D (2002) Natural organic matter affects arsenic speciation and sorption onto hematite. Environ Sci Technol 36(13):2889–2896
Schijven JF, ŠimŮnek J (2002) Kinetic modeling of virus transport at the field scale. J Contam Hydrol 55(1–2):113–135
Schulman JH (1960) Colloid chemistry. Annu Rev Phys Chem 11(1):169–186
Simunek J, van Genuchten MT, Sejna M (2016) Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J 15(7). https://doi.org/10.2136/vzj2016.04.0033
Solovitch N, Labille J, Rose J, Chaurand P, Borchneck D, Wiecner MR, Bottero JY (2010) Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ Sci Technol 44(13):4897–4902
Svecova L, Cremel S, Sirguey C, Simonnot MO (2008) Comparison between batch and column experiments to determine the surface charge properties of rutile TiO2 powder. J Colloid Interface Sci 325(2):363–370
Toloni I, Lehmann F, Ackerer P (2014) Modeling the effects of water velocity on TiO2 nanoparticles transport in saturated porous media. J Contam Hydrol 171:42–48
Tufenkji N, Elimelech M (2005) Breakdown of colloid filtration theory: role of the secondary energy minimum and surface charge heterogeneities. Langmuir 21(3):841–852
USEPA (2007) Nanotechnology white paper. Prepared for the U.S. Environmental Protection Agency by members of the nanotechnology workgroup, a group of EPA’s science policy council science policy council. U.S. Environmental Protection Agency, Washington, DC
Wang C, Bobba AD, Attinti R, Lazouskaya V, Wang LP, ** Y (2012a) Retention and transport of silica nanoparticles in saturated porous media: effect of concentration and particle size. Environ Sci Technol 46(13):7151–7158
Wang D, Bradford SA, Harvey RW, Gao B, Cang L, Zhou D (2012b) Humic acid facilitates the transport of ARS-labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ Sci Technol 46(5):2738–2745
Wang D, Zhang W, Zhou D (2013) Antagonistic effects of humic acid and iron oxyhydroxide grain-coating on biochar nanoparticle transport in saturated sand. Environ Sci Technol 47(10):5154–5161
Xu X, Xu N, Cheng X, Guo P, Chen Z, Wang D (2017) Transport and aggregation of rutile titanium dioxide nanoparticles in saturated porous media in the presence of ammonium. Chemosphere 169:9–17
Xu N, Cheng X, Zhou K, Xu X, Li Z, Chen J (2018) Facilitated transport of titanium dioxide nanoparticles via hydrochars in the presence of ammonium in saturated sands: effects of pH, ionic strength, and ionic composition. Sci Total Environ 612:1348–1357
Yuan J, **ng W, Gu G, Wu L (2008) The properties of organic pigment encapsulated with nano-silica via layer-by-layer assembly technique. Dyes Pigments 76(2):463–469
Zhang W, Morales VL, Cakmak ME, Salvucci AE, Geohring LD, Hay AG, Parlange JY, Steenhuis TS (2010) Colloid transport and retention in unsaturated porous media: effect of colloid input concentration. Environ Sci Technol 44(13):4965–4972
Zhu Z, Andelman T, Yin M, Chen T, Ehrlich S, O’Brien S (2005) Synchrotron x-ray scattering of Zno nanorods: periodic ordering and lattice size. J Mater Res 20(4):1033–1041
Acknowledgments
We also greatly appreciate the support from the Jiangsu Collaborative Innovation Center of Technology and Material for Water Treatment.
Funding
This research was funded by the National Natural Science Foundation of China (grant nos. 21777110 and 21377090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 95 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Li, D., Ye, Z. et al. Effect of humic acid on the sedimentation and transport of nanoparticles silica in water-saturated porous media. J Soils Sediments 20, 911–920 (2020). https://doi.org/10.1007/s11368-019-02444-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02444-x