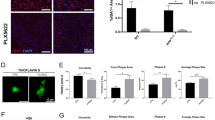
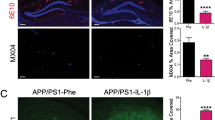
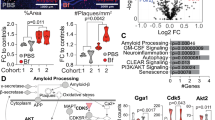
Abstract
Terminal sialic acid residues are present on most glycoproteins and glycolipids, but levels of sialylation are known to change in the brain throughout the lifespan as well as during disease. Sialic acids are important for numerous cellular processes including cell adhesion, neurodevelopment, and immune regulation as well as pathogen invasion into host cells. Neuraminidase enzymes, also known as sialidases, are responsible for removal of terminal sialic acids in a process known as desialylation. Neuraminidase 1 (Neu1) cleaves the α-2,6 bond of terminal sialic acids. Aging individuals with dementia are often treated with the antiviral medication oseltamivir, which is associated with induction of adverse neuropsychiatric side effects; this drug inhibits both viral and mammalian Neu1. The present study tested whether a clinically relevant antiviral dosing regimen of oseltamivir would disrupt behavior in the 5XFAD mouse model of Alzheimer’s disease amyloid pathology or wild-type littermates. While oseltamivir treatment did not impact mouse behavior or modify amyloid plaque size or morphology, a novel spatial distribution of α-2,6 sialic acid residues was discovered in 5XFAD mice that was not present in wild-type littermates. Further analyses revealed that α-2,6 sialic acid residues were not localized the amyloid plaques but instead localized to plaque-associated microglia. Notably, treatment with oseltamivir did not alter α-2,6 sialic acid distribution on plaque-associated microglia in 5XFAD mice which may be due to downregulation of Neu1 transcript levels in 5XFAD mice. Overall, this study suggests that plaque-associated microglia are highly sialylated and are resistant to change with oseltamivir, thus interfering with microglia immune recognition of and response to amyloid pathology.






Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Neu1:
-
neuraminidase 1
- AD:
-
Alzheimer’s Disease
- 5XFAD:
-
Five familiar Alzheimer’s disease mutations
- Aβ:
-
amyloid beta
- PTM:
-
post-translational modification
- SA:
-
sialic acid
- Siglec:
-
sialic acid binding immunoglobulin-like lectin
- CSF:
-
cerebral spinal fluid
- MCI:
-
mild cognitive impairment
- MS:
-
mass spectrometry
- APP:
-
amyloid precursor protein
- WT:
-
wild-type
- PCR:
-
polymerase chain reaction
- OF:
-
open field
- NOR:
-
novel object recognition
- DI:
-
discrimination index
- IHC:
-
immunohistochemistry
- IF:
-
immunofluorescence
- ROI:
-
region of interest
References
Aires V, Coulon-Bainier C, Pavlovic A, Ebeling M, Schmucki R, Schweitzer C, Kueng E, Gutbier S, Harde E. CD22 blockage restores age-related impairments of microglia surveillance capacity. Front Immunol. 2021;0:2096.
Allendorf DH, Puigdellívol M, Brown GC. Activated microglia desialylate their surface, stimulating complement receptor 3-mediated phagocytosis of neurons. Glia. 2020;68:989–98.
Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E, Nixon R, D’Azzo A. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat Commun. 2013;4:2734.
Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol. 2017;147:149–74.
Bowman K, Jones L, Masoli J, Mujica-Mota R, Strain D, Butchart J, Valderas JM, Fortinsky RH, Melzer D, Delgado J. Predicting incident delirium diagnoses using data from primary-care electronic health records. Age Ageing. 2020;49:374–381. Available at: https://academic.oup.com/ageing/article/49/3/374/5814887. Accessed 26 Aug 2022.
Chen R, Fang Z, Huang Y. Neuropsychiatric events in an adult patient with influenza a (H3N2) treated with oseltamivir (Tamiflu): A case report. BMC Infect Dis. 2019;19:4–7.
Cowan CB, Patel DA, Good TA. Exploring the mechanism of β-amyloid toxicity attenuation by multivalent sialic acid polymers through the use of mathematical models. J Theor Biol. 2009;258:189–97.
Crain SM, Shen KF. Neuraminidase inhibitor, oseltamivir blocks GM1 ganglioside-regulated excitatory opioid receptor-mediated hyperalgesia, enhances opioid analgesia and attenuates tolerance in mice. Brain Res. 2004;995:260–266. Available at: https://pubmed.ncbi.nlm.nih.gov/14672816/. Accessed 14 Oct 2022.
De Oliveira JT, Santos AL, Gomes C, Barros R, Ribeiro C, Mendes N, De Matos AJ, Vasconcelos MH, Oliveira MJ, Reis CA, Gärtner F. Anti-influenza neuraminidase inhibitor oseltamivir phosphate induces canine mammary cancer cell aggressiveness. PLoS One. 2015;10:e0121590. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0121590. Accessed 19 Oct 2022.
Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–395. Available at: https://pubmed.ncbi.nlm.nih.gov/31986070/. Accessed 26 Aug 2022.
El-Sayed WM, Al-Kahtani MA. Potential adverse effects of oseltamivir in rats: males are more vulnerable than females. https://doi.org/10.1139/y11-060 2011;89:623–630. Available at: https://cdnsciencepub.com/doi/10.1139/y11-060. Accessed 14 Oct 2022.
Forner S, et al. Systematic phenoty** and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci Data. 2021;8(1):1–16 Available at: https://www.nature.com/articles/s41597-021-01054-y. Accessed 19 Oct 2022.
Galleguillos D, Wang Q, Steinberg N, Zaidi A, Shrivastava G, Dhami K, Daskhan GC, Schmidt EN, Dworsky-Fried Z, Giuliani F, Churchward M, Power C, Todd K, Taylor A, Macauley MS, Sipione S. Anti-inflammatory role of GM1 and other gangliosides on microglia. J Neuroinflammation. 2022;19. Available at: https://pmc/articles/PMC8739653/. Accessed 16 Feb 2023.
Gupta YK, Meenu M, Mohan P. The Tamiflu fiasco and lessons learnt. Indian J Pharmacol. 2015;47:11–6.
Hajjar I, Liu C, Jones DP, Uppal K. Untargeted metabolomics reveal dysregulations in sugar, methionine, and tyrosine pathways in the prodromal state of AD. 2020. Available at: https://doi.org/10.1002/dad2.12064. Accessed 10 Sept 2021.
Hama R. The mechanisms of delayed onset type adverse reactions to oseltamivir. Infect Dis (London, England). 2016;48:651. Available at: https://pmc/articles/PMC4973146/. Accessed 5 Dec 2021.
Hatori A, Arai T, Yanamoto K, Yamasaki T, Kawamura K, Yui J, Konno F, Nakao R, Suzuki K, Zhang MR. Biodistribution and metabolism of the anti-influenza drug [11C]oseltamivir and its active metabolite [11C]Ro 64–0802 in mice. Nucl Med Biol. 2009;36:47–55.
Ikeda A, Komamizu M, Hayashi A, Yamasaki C, Okada K, Kawabe M, Komatsu M, Shiozaki K. Neu1 deficiency induces abnormal emotional behavior in zebrafish. Sci Rep. 2021;11(1):1–15 Available at: https://www.nature.com/articles/s41598-021-92778-9. Accessed 19 Oct 2022.
Ison MG, Portsmouth S, Yoshida Y, Shishido T, Mitchener M, Tsuchiya K, Uehara T, Hayden FG. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20:1204–1214. Available at: http://www.thelancet.com/article/S1473309920300049/fulltext. Accessed 25 Aug 2022.
Izumi Y, Tokuda K, O’Dell KA, Zorumski CF, Narahashi T. Synaptic and behavioral interactions of oseltamivir (Tamiflu) with neurostimulants. Hum Exp Toxicol. 2008;27:911–917. Available at: https://pubmed.ncbi.nlm.nih.gov/19273546/. Accessed 12 Dec 2021.
Klaus C, Hansen JN, Ginolhac A, Gérard D, Gnanapragassam VS, Horstkorte R, Rossdam C, Buettner FFR, Sauter T, Sinkkonen L, Neumann H, Linnartz-Gerlach B. Reduced sialylation triggers homeostatic synapse and neuronal loss in middle-aged mice. Neurobiol Aging. 2020;88:91–107. Available at: https://doi.org/10.1016/j.neurobiolaging.2020.01.008.
Klaus C, Liao H, Allendorf DH, Brown GC, Neumann H. Sialylation acts as a checkpoint for innate immune responses in the central nervous system. Glia. 2021;69:1619–36.
Li F, Ding J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell. 2019;10:550–565. Available at: https://doi.org/10.1007/s13238-018-0597-5.
Liao H, Klaus C, Neumann H. Control of innate immunity by sialic acids in the nervous tissue. Int J Mol Sci. 2020;21:5494. Available at: https://www.mdpi.com/1422-0067/21/15/5494/htm. Accessed 22 Aug 2022.
Liao H, Winkler J, Wißfeld J, Shahraz A, Klaus C, Neumann H. Low molecular weight polysialic acid prevents lipopolysaccharide-induced inflammatory dopaminergic neurodegeneration in humanized SIGLEC11 transgenic mice. Glia. 2021. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/glia.24073. Accessed 20 Aug 2021.
Linnartz-Gerlach B, Kopatz J, Neumann H. Siglec functions of microglia. Glycobiology. 2014;24:794–799. Available at: https://pubmed.ncbi.nlm.nih.gov/24833613/. Accessed 20 Aug 2021.
Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol Psychiatry. 1997;41:1131–6.
Marino JH, Tan C, Davis B, Han ES, Hickey M, Naukam R, Taylor A, Miller KS, Van De Wiele CJ, Teague TK. Disruption of thymopoiesis in ST6Gal I-deficient mice. Glycobiology. 2008;18:719–26.
McElhaney JE, Verschoor CP, Andrew MK, Haynes L, Kuchel GA, Pawelec G. The immune response to influenza in older humans: beyond immune senescence. Immun Ageing. 2020;17. Available at: https://pmc/articles/PMC7204009/. Accessed 18 July 2022.
Morimoto K, Nakakariya M, Shirasaka Y, Kakinuma C, Fujita T, Tamai I, Ogihara T. Oseltamivir (Tamiflu) efflux transport at the blood-brain barrier via P-glycoprotein. Drug Metab Dispos. 2008;36:6–9. Available at: https://dmd.aspetjournals.org/content/36/1/6. Accessed 14 Oct 2022.
Nagamine S, Yamazaki T, Makioka K, Fujita Y, Ikeda M, Takatama M, Okamoto K, Yokoo H, Ikeda Y. Hypersialylation is a common feature of neurofibrillary tangles and granulovacuolar degenerations in Alzheimer’s disease and tauopathy brains. Neuropathology. 2016;36:333–345. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/neup.12277. Accessed 19 Aug 2022.
Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;435 43:436–441. Available at: https://www.nature.com/articles/ng.801. Accessed 12 Jan 2023.
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. Available at: https://pubmed.ncbi.nlm.nih.gov/17021169/. Accessed 26 Aug 2022.
Oblak AL, et al. Comprehensive evaluation of the 5XFAD mouse model for preclinical testing applications: a MODEL-AD study. Front Aging Neurosci. 2021;13. Available at: https://pmc/articles/PMC8346252/. Accessed 18 Apr 2022.
Palmigiano A, Barone R, Sturiale L, Sanfilippo C, Bua RO, Romeo DA, Messina A, Capuana ML, Maci T, Le Pira F, Zappia M, Garozzo D. CSF N-glycoproteomics for early diagnosis in Alzheimer’s disease. J Proteomics. 2016;131:29–37.
Peng W, Paulson JC. CD22 ligands on a natural N-glycan scaffold efficiently deliver toxins to B-lymphoma cells. J Am Chem Soc. 2017;139:12450–8.
Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, Li L, Lee DP, Morgens DW, Yang AC, Shuken SR, Gate D, Scott M, Khatri P, Luo J, Bertozzi CR, Bassik MC, Wyss-Coray T. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nat. 2019;5687751 568:187–192. Available at: https://www.nature.com/articles/s41586-019-1088-4. Accessed 16 Aug 2021.
Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–59.
Pshezhetsky AV, Ashmarina M. Kee** it trim: roles of neuraminidases in CNS function. Glycoconj J. 2018;35:375. Available at: https://pmc/articles/PMC6182584/. Accessed 15 Apr 2022.
Rawal P, Zhao L. Sialometabolism in brain health and Alzheimer’sd. Front Neurosci. 2021;15:1–19.
Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518.
Siddiqui SS, Springer SA, Verhagen A, Sundaramurthy V, Alisson-Silva F, Jiang W, Ghosh P, Varki A. The Alzheimer’s disease-protective CD33 splice variant mediates adaptive loss of function via diversion to an intracellular pool. J Biol Chem. 2017;292:15312–15320. Available at: https://pubmed.ncbi.nlm.nih.gov/28747436/. Accessed 31 Aug 2021.
Siddiqui SS, Matar R, Merheb M, Hodeify R, Vazhappilly CG, Marton J, Shamsuddin SA, Zouabi HA. Siglecs in brain function and neurological disorders. Cells. 2019;8. Available at: https://pubmed.ncbi.nlm.nih.gov/31546700/. Accessed 13 Aug 2021.
Sur M, Lopez MJ, Baker MB. Oseltamivir. Kucers Use Antibiot A Clin Rev Antibacterial, Antifung Antiparasit Antivir Drugs, Seventh Ed:4580–4609. 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK539909/. Accessed 26 Aug 2022.
Ton Tran HT, Li C, Chakraberty R, Cairo CW. NEU1 and NEU3 enzymes alter CD22 organization on B cells. Biophys Rep. 2022;2(3):100064.
Toovey S, Rayner C, Prinssen E, Chu T, Donner B, Thakrar B, Dutkowski R, Hoffmann G, Breidenbach A, Lindemann L, Carey E, Boak L, Gieschke R, Sacks S, Solsky J, Small I, Reddy D. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir. Drug Saf. 2012;2008 3112 31:1097–1114. Available at: https://springer.longhoe.net/article/10.2165/0002018-200831120-00006. Accessed 18 July 2022.
Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351. Available at: https://pmc/articles/PMC2553044/. Accessed 13 Aug 2021.
Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Investig. 2007;879 87:851–857. Available at: https://www.nature.com/articles/3700656. Accessed 13 Aug 2021.
Walker JA, Smith KGC. CD22: an inhibitory enigma. Immunology. 2008;123:314. Available at: https://pmc/articles/PMC2433339/. Accessed 10 Sept 2021.
Walker DG, Whetzel AM, Serrano G, Sue LI, Beach TG, Lue LF. Association of CD33 polymorphism rs3865444 with Alzheimer’s disease pathology and CD33 expression in human cerebral cortex. Neurobiol Aging. 2015;36:571–82.
Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu®) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother. 2005;55:i5–i21. Available at: https://academic.oup.com/jac/article/55/suppl_1/i5/2473509. Accessed 6 Jan 2023.
Wei M, Wang PG. Desialylation in physiological and pathological processes: new target for diagnostic and therapeutic development. Prog Mol Biol Transl Sci. 2019;162:25–57.
Wong ZX, Jones JE, Anderson GP, Gualano RC. Oseltamivir treatment of mice before or after mild influenza infection reduced cellular and cytokine inflammation in the lung. Influenza Other Respi Viruses. 2011;5:343. Available at: https://pmc/articles/PMC4942046/. Accessed 26 Aug 2022.
Yang K, Yang Z, Chen X, Li W. The significance of sialylation on the pathogenesis of Alzheimer’s disease. Brain Res Bull. 2021;173:116–123. Available at: https://doi.org/10.1016/j.brainresbull.2021.05.009.
Acknowledgements
The authors would like to thank Mallory Maybrier for histological expertise and Kathleen Bensen for cell counting.
Funding
This work was supported by The National Institutes of Health [T32AG021890 to CF, NS082145 to KFB, R21AG072423 and pilot funding under P30AG013319 to SCH, and P30AG066546 to KFB], the Texas Alzheimer’s Research and Care Consortium to KFB, and the Bartell and Mollie Zachry Endowment for Alzheimer’s Research and Patient Care to KFB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declre no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplemental Fig. 1
(PNG 196 kb)

Supplemental Fig. 2
(PNG 3507 kb)
Supplement 1
(TXT 2 kb)
About this article
Cite this article
Fastenau, C., Wickline, J.L., Smith, S. et al. Increased α-2,6 sialic acid on microglia in amyloid pathology is resistant to oseltamivir. GeroScience 45, 1539–1555 (2023). https://doi.org/10.1007/s11357-023-00761-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00761-1