Abstract
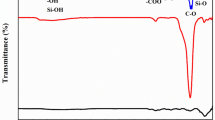
Finding a cost-effective, efficient, and environmentally friendly technique for the removal of mercury ion (Hg2+) in water and wastewater can be a challenging task. This paper presents a novel and efficient adsorbent known as the graphene oxide-Cu2SnS3-polyaniline (GO-CTS-PANI) nanocomposite, which was synthesised and utilised to eliminate Hg2+ from water samples. The soft–soft interaction between Hg2+ and sulphur atoms besides chelating interaction between -N and Hg2+ is the main mechanism for Hg2+ adsorption onto the GO-CTS-PANI adsorbent. Various characterisation techniques, including Fourier transform infrared spectrophotometry (FT-IR), field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), elemental map** analysis, and X-ray diffraction analysis (XRD), were employed to analyse the adsorbent. The Box-Behnken method, utilising Design Expert Version 7.0.0, was employed to optimise the crucial factors influencing the adsorption process, such as pH, adsorbent quantity, and contact time. The results indicated that the most efficient adsorption occurred at pH 6.5, with 12 mg of GO-CTS-PANI adsorbent, and 30-min contact time that results in a maximum removal rate of 95% for 50 mg/L Hg2+ ions. The study also investigated the isotherm and kinetics of the adsorption process that the adsorption of Hg2+ onto the adsorbent happened in sequential layers (Freundlich isotherm) and followed by the pseudo-second-order kinetic model. Furthermore, response surface methodology (RSM) analysis indicates that pH is the most influential parameter in enhancing adsorption efficiency. In addition to traditional models, this study employed some artificial intelligence (AI) methods including the Random Forest algorithm to enhance the prediction of adsorption process efficiency. The findings demonstrated that the Random Forest algorithm exhibited high accuracy with a correlation coefficient of 0.98 between actual and predicted adsorption rates. This study highlights the potential of the GO-CTS-PANI nanocomposite for effectively removing of Hg2+ ions from water resources.
























Similar content being viewed by others
Data availability
All data comprised within the study are available upon request.
References
Abu Ismaiel A, KheireddineAroua M, Yusoff R (2013) Palm shell activated carbon impregnated with task-specific ionic-liquids as a novel adsorbent for the removal of mercury from contaminated water. Chem Eng J 225:306–314
Albatrni H, Qiblawey H, El-Naas MH (2021) Comparative study between adsorption and membrane technologies for the removal of mercury. Sep Purif Technol 257:117833
Amini-Fazl MS, Barzegarzadeh M, Mohammadi R (2021) Surface modification of graphene oxide with crosslinked polymethacrylamide via RAFT polymerization strategy: effective removal of heavy metals from aqueous solutions. J Inorg Organomet Polym 31:2959–2970
Anirudhan TS, Shainy F (2015) Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. J Colloid Interface Sci 456:22–31
Arshad F, Selvaraj M, Zain J, Banat F, Abu Haija M (2019) Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep Purif Technol 209:870–880
Awad FS, AbouZied KM, Abou El-Maaty WM, El-Wakil AM, Samy El-Shall M (2020) Effective removal of mercury(II) from aqueous solutions by chemically modified graphene oxide nanosheets. Arab J Chem 13:2659–2670
Babel V, Lal Hiran B (2021) A review on polyaniline composites: synthesis, characterization, and applications. Polym Compos 42:3142–3157
Berg DM, Djemour R, Gütay L, Zoppi G, Siebentritt S, Dale PJ (2012) Thin film solar cells based on the ternary compound Cu2SnS3. Thin Solid Films 520:6291–6294
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Eftekhari M, Akrami M, Gheibi M, Azizi-Toupkanloo H, Fathollahi-Fard AM, Tian G (2020) Cadmium and copper heavy metal treatment from water resources by high performance folic acid-graphene oxide nanocomposite adsorbent and evaluation of adsorptive mechanism using computational intelligence, isotherm, kinetic, and thermodynamic analyses. Environ Sci Pollut Res 27:43999–44021
Eftekhari M, Gheibi M, Azizi-Toupkanloo H, Hossein-Abadi Z, Khraisheh M, Fathollahi-Fard AM, Tian G (2021) Statistical optimization, soft computing prediction, mechanistic and empirical evaluation for fundamental appraisal of copper, lead and malachite green adsorption. J Ind Inf Integr 23:100219
Gao P, Lei J, Tan J, Wang G, Liu H, Zhou L (2021) Self-assembled magnetic microcrystalline cellulose/MoS2/Fe3O4 composite for efficient adsorptive removal of mercury ions (Hg2+). Compos Commun 25:100736
Ghadirimoghaddam D, Gheibi M, Eftekhari M (2023) Graphene oxide-cyanuric acid nanocomposite as a novel adsorbent for highly efficient solid phase extraction of Pb2+ followed by electrothermal atomic absorption spectrometry; statistical, soft computing and mechanistic efforts. Inter J Environ Anal Chem 103:469–490
UN Environment, 2019. Global Mercury Assessment 2018. UN Environment Programme, Chemicals and Health Branch Geneva, Switzerland
Han Z, Guo Y, Yang W, Tang R, Wang H, Wu S (2020) Removal of mercury from flue gases over iron modified activated carbon made by in situ ion exchange method. J Energy Inst 93:1411–1418
Homayoon F, Faghihian H, Torki F (2017) Application of a novel magnetic carbon nanotube adsorbent for removal of mercury from aqueous solutions. Environ Sci Pollut Res 24:11764–11778
Jathar SB, Rondiya SR, Jadhav YA, Nilegave DS, Cross RW et al (2021) Ternary Cu2SnS3: synthesis, structure, photoelectrochemical activity, and heterojunction band offset and alignment. Chem Mater 33(6):1983–1993
Krishna Kumar AS, Kalidhasan S, Rajesh V, Rajesh N (2013) Adsorptive demercuration by virtue of an appealing interaction involving biopolymer cellulose and mercaptobenzothiazole. Ind Eng Chem Res 52:11838–11849
Lei Y, Chen F, Luo Y, Zhang L (2014) Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions. Chem Phys Lett 593:122–127
Li R, Liu L, Yang F (2013) Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg(II). Chem Eng J 229:460–468
Li L, Luo C, Li X, Duan H, Wang X (2014) Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int J Biol Macromol 66:172–178
Liu J, Duan Y, Song L, Zhang X (2018) Constructing sandwich-like polyaniline/graphene oxide composites with tunable conjugation length toward enhanced microwave absorption. Org Electron 63:175–183
Mbanga O, Ncube S, Tutu H, Chimuka L, Cukrowska E (2019) Mercury accumulation and biotransportation in wetland biota affected by gold mining. Environ Monit Assess 191:186
Olatunde OC, Onwudiwe DC (2022) Synthesis of reduced graphene oxide/copper tin sulfide (Cu2SnS3) composite for the photocatalytic degradation of tetracycline. J Inorg Organomet Polym 32:2578–2590
Raj D, Maiti SK (2019) Sources, toxicity, and remediation of mercury: an essence review. Environ Monit Assess 191:566
Rezazadeh N, Danesh S, Eftekhari M, Farahmandzadeh M (2022) Application of graphene oxide and its derivatives on the adsorption of a cationic surfactant (interaction mechanism, kinetic, isotherm curves and thermodynamic studies). J Mol Liq 368:120720
Rice KM, Walker EM, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83
Saadati T, Eftekhari M, Rezazadeh N, Nazarabad MK (2023) Graphene oxide–bismuth tungstate (GO–Bi2WO6) nanocomposite as a green adsorbent for lead removal: isotherm, kinetics and thermodynamic study. Int J Environ Sci Technol 20:1301–1314
Sahan T, Erol F, Yilmaz S (2018) Mercury(II) adsorption by a novel adsorbent mercapto-modified bentonite using ICP-OES and use of response surface methodology for optimization. Microchim J 138:360–368
Santana AJ, dos Santos WNL, Silva LOB, das Virgens CF (2016) Removal of mercury(II) ions in aqueous solution using the peel biomass of Pachira aquatica Aubl: kinetics and adsorption equilibrium studies. Environ Monit Assess 188:293
Santhana Krishna Kumar A, Kalidhasan S, Rajesh V, Rajesh N (2013) Adsorptive demercuration by virtue of an appealing interaction involving biopolymer cellulose and mercaptobenzothiazole. Ind Eng Chem Res 52:11838–11849
Shabani-Nooshabadi M, Zahedi F (2017) Electrochemical reduced graphene oxide-polyaniline as effective nanocomposite film for high-performance supercapacitor applications. Electrochim Acta 245:575–586
Shi L, Wang W, Wu C, Ding J, Li Q (2017) Synthesis of Cu2SnS3 nanosheets as an anode material for sodium ion batteries. J Alloys Compd 699:517–520
Streets DG, Devane MK, Lu Z, Bond TC, Sunderland EM, Jacob DJ (2011) All-time releases of mercury to the atmosphere from human activities. Environ Sci Technol 45:10485–10491
Suryawanshi P, Babar B, Mohite A, Pawar U, Bhosale A, Shelke HA (2020) Simple chemical approach for the deposition of Cu2SnS3 (CTS) thin films. Mater Today Proc 43:2682–2688
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
Vasudevan S, Lakshmi J, Sozhan G (2012) Optimization of electrocoagulation process for the simultaneous removal of mercury, lead, and nickel from contaminated water. Environ Sci Pollut Res 19:2734–2744
Velempini T, Pillay K (2019) Sulphur functionalized materials for Hg(II) adsorption: a review. J Environ Chem Eng 7:103350
Wang C, Tian H, Jiang J, Zhou T, Zeng Q, He X, Huang P, Yao Y (2017) Facile synthesis of different morphologies of Cu2SnS3 for high-performance supercapacitors. ACS Appl Mater Interfaces 9(31):26038–26044
Wei Y, Zhang Y, Gao X, Ma Z, Wang X, Gao C (2018) Multilayered graphene oxide membranes for water treatment: a review. Carbon 139:964–981
Yan X, Feng J, Li P, Li J, Ren B, Gao S, Cao R (2021) Fast and efficient removal of mercury ions using zirconium-based metal–organic framework filter membranes. Inorg Chem Commun 131:108796
Yap PL, Tung TT, Kabiri S, Matulick N, Tran DNH, Losic D (2020) Polyamine-modified reduced graphene oxide: a new and cost-effective adsorbent for efficient removal of mercury in waters. Sep Purif Technol 238:116441
Yu JG, Yue BY, Wu XW, Liu Q, Jiao FP, Jiang XY, Chen XQ (2016) Removal of mercury by adsorption: a review. Environ Sci Pollut Res 23:5056–5076
Zaman MB, Poolla R (2020) Morphological tuning of hydrothermally derived visible light active Cu2SnS3 nanostructures and their applications in photocatalytic degradation of reactive industrial dyes. Opt Mater 104:109853
Zeng H, Wang L, Zhang D, Yan P, Nie J, Sharma VK, Wang C (2019) Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem Engin J 358:253–263
Author information
Authors and Affiliations
Contributions
Sara Enferadi: data curation, methodology; Mohammad Eftekhari: data curation, methodology, supervision, conceptualisation, writing—review and editing; Mohammad Gheibi: methodology, software, writing—review and editing; Nikoo Nabizadeh Moghaddam: data curation, methodology; Stanislaw Wacławek: writing—review and editing, and supervision; Kourosh Behzadian: data curation and conceptualisation, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enferadi, S., Eftekhari, M., Gheibi, M. et al. Modelling and optimising the performance of graphene oxide-Cu2SnS3-polyaniline nanocomposite as an adsorbent for mercury ion removal. Environ Sci Pollut Res 31, 38196–38216 (2024). https://doi.org/10.1007/s11356-024-33746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33746-4