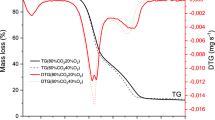
Abstract
Biomass as a raw material has profound implications for thermal conversion processes. It is important to study the relationship between kinetic modeling to depict significant importance in thermal processing by estimating volatile yield and reaction performance during biomass decomposition. This work aimed to determine the thermal decomposition reaction kinetics of non-woody (oil palm trunk (OPT)) and woody (rubberwood sawdust (RWS)) biomass. Devolatilization of biomass is determined by the thermogravimetric analysis (TGA) at three different heating rates (10, 20, and 30 °C/min) using nitrogen as inert gas. The kinetic analysis used isoconversion models of Friedman, Ozawa-Flynn-Wall (OFW), and Kissinger–Akahira–Sunose (KAS). The activation energy varied from 218.4 to 303.8 kJ/mol (Friedman), 235.9 to 299.1 kJ/mol (OFW), and 235.8 to 298.9 kJ/mol (KAS) for OPT; and 199.7 to 228.1 kJ/mol (Friedman), 210.6 to 225.6 kJ/mol (OFW), and 210.7 to 225.2 kJ/mol (KAS) for RWS. The kinetic analysis indicated that RWS and OPT had diverse reaction kinetics, which depend on the reaction rate and order of the reaction. Experimental and theoretical conversion data agreed reasonably well, indicating that these results can be used for future OPT and RWS process modeling. Consistency of results is validated using GC–MS equipped with a pyrolyzer.
Graphical Abstract





Similar content being viewed by others
Data availability
All data given in either table or figure.
Code availability
Not applicable.
References
Alqarni AO, Nabi RAU, Althobiani F, et al (2022) Statistical optimization of pyrolysis process for thermal destruction of plastic waste based on temperature-dependent activation energies and pre-exponential factors. Processes 10. https://doi.org/10.3390/pr10081559
Alwani MS, Abdul Khalil HPS, Sulaiman O et al (2014) An approach to using agricultural waste fibres in biocomposites application: thermogravimetric analysis and activation energy study. BioResources 9:218–230. https://doi.org/10.15376/biores.9.1.218-230
Anand V, Gautam R, Vinu R (2017) Non-catalytic and catalytic fast pyrolysis of Schizochytrium limacinum microalga. Fuel 205:1–10. https://doi.org/10.1016/j.fuel.2017.05.049
Biswas B, Pandey N, Bisht Y et al (2017) Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63. https://doi.org/10.1016/j.biortech.2017.02.046
Brar JS, Singh K, Wang J, Kumar S (2012) Cogasification of coal and biomass: a review. Int J for Res 2012:1–10. https://doi.org/10.1155/2012/363058
Ceylan S, Topçu Y (2014) Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour Technol 156:182–188. https://doi.org/10.1016/j.biortech.2014.01.040
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143. https://doi.org/10.1021/es8002684
Chen L, Wang X, Yang H et al (2015) Study on pyrolysis behaviors of non-woody lignins with TG-FTIR and Py-GC/MS. J Anal Appl Pyrolysis 113:499–507. https://doi.org/10.1016/j.jaap.2015.03.018
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A (2011) Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol 102:6230–6238. https://doi.org/10.1016/j.biortech.2011.02.060
Du J, Dou B, Zhang H et al (2023) Non-isothermal kinetics of biomass waste pyrolysis by TG-MS/DSC. Carbon Capture Sci Technol 6:100097. https://doi.org/10.1016/j.ccst.2023.100097
Gai C, Dong Y, Zhang T (2013) The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour Technol 127:298–305. https://doi.org/10.1016/j.biortech.2012.09.089
Gautam R, Vinu R (2018) Non-catalytic fast pyrolysis and catalytic fast pyrolysis of Nannochloropsis oculata using Co-Mo/γ-Al2O3 catalyst for valuable chemicals. Algal Res 34:12–24. https://doi.org/10.1016/j.algal.2018.06.024
Heydari M, Rahman M, Gupta R (2015) Kinetic study and thermal decomposition behavior of lignite coal. Int J Chem Eng 2015:1–9. https://doi.org/10.1155/2015/481739
Hu X, Gholizadeh M (2019) Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage. J Energy Chem 39:109–143. https://doi.org/10.1016/j.jechem.2019.01.024
Huang X, Cao J-P, Zhao X-Y et al (2016) Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 169:93–98. https://doi.org/10.1016/j.fuel.2015.12.011
Li J, Dou B, Zhang H et al (2021) Pyrolysis characteristics and non-isothermal kinetics of waste wood biomass. Energy 226:120358. https://doi.org/10.1016/j.energy.2021.120358
Liaw S-S, Haber Perez V, Zhou S et al (2014) Py-GC/MS studies and principal component analysis to evaluate the impact of feedstock and temperature on the distribution of products during fast pyrolysis. J Anal Appl Pyrolysis 109:140–151. https://doi.org/10.1016/j.jaap.2014.06.018
Loh SK (2017) The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers Manag 141:285–298
Ma M, Xu D, Zhi Y et al (2022) Co-pyrolysis re-use of sludge and biomass waste: development, kinetics, synergistic mechanism and industrialization. J Anal Appl Pyrolysis 168:105746. https://doi.org/10.1016/j.jaap.2022.105746
Maiti S, Purakayastha S, Ghosh B (2007) Thermal characterization of mustard straw and stalk in nitrogen at different heating rates. Fuel 86:1513–1518. https://doi.org/10.1016/j.fuel.2006.11.016
McKendry P (2002a) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Mckendry P (2002b) Energy production from biomass (part 2): conversion technologies. Bioresour Technol 83:47–54. https://doi.org/10.1016/S0960-8524(01)00119-5
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74. https://doi.org/10.1016/j.biortech.2017.12.029
Mishra RK, Mohanty K (2019) Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-019-00469-3
Mishra RK, Mohanty K (2020) Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers Biorefin 10:621–637. https://doi.org/10.1007/s13399-019-00469-3
Mishra RK, Sahoo A, Mohanty K (2019) Pyrolysis kinetics and synergistic effect in co-pyrolysis of Samanea saman seeds and polyethylene terephthalate using thermogravimetric analyser. Bioresour Technol 289:121608. https://doi.org/10.1016/j.biortech.2019.121608
Mishra RK, Kumar V, Mohanty K (2020) Pyrolysis kinetics behaviour and thermal pyrolysis of Samanea saman seeds towards the production of renewable fuel. J Energy Inst 93:1148–1162. https://doi.org/10.1016/j.joei.2019.10.008
Mishra A, Nanda S, RanjanParida M et al (2023) A comparative study on pyrolysis kinetics and thermodynamic parameters of little millet and sunflower stems biomass using thermogravimetric analysis. Bioresour Technol 367:128231. https://doi.org/10.1016/j.biortech.2022.128231
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20:848–889. https://doi.org/10.1021/ef0502397
Ojha DK, Viju D, Vinu R (2021) Fast pyrolysis kinetics of lignocellulosic biomass of varying compositions. Energy Convers Manag X 10:100071. https://doi.org/10.1016/J.ECMX.2020.100071
Palamanit A, Khongphakdi P, Tirawanichakul Y, Phusunti N (2019) Investigation of yields and qualities of pyrolysis products obtained from oil palm biomass using an agitated bed pyrolysis reactor. Biofuel Res J 6:1065–1079. https://doi.org/10.18331/BRJ2019.6.4.3
Pattanayak S, Hauchhum L, Loha C et al (2021) Experimental investigation on pyrolysis kinetics, reaction mechanisms and thermodynamic parameters of biomass and tar in N2 atmosphere. Sustain Energy Technol Assess 48:101632. https://doi.org/10.1016/j.seta.2021.101632
Pattiya A, Suttibak S (2012a) Production of bio-oil via fast pyrolysis of agricultural residues from cassava plantations in a fluidised-bed reactor with a hot vapour filtration unit. J Anal Appl Pyrolysis 95:227–235. https://doi.org/10.1016/j.jaap.2012.02.010
Pattiya A, Suttibak S (2012b) Influence of a glass wool hot vapour filter on yields and properties of bio-oil derived from rapid pyrolysis of paddy residues. Bioresour Technol 116:107–113. https://doi.org/10.1016/j.biortech.2012.03.116
Peterson CA, Hornbuckle MK, Brown RC (2022) Biomass pyrolysis devolatilization kinetics of herbaceous and woody feedstocks. Fuel Process Technol 226:107068. https://doi.org/10.1016/j.fuproc.2021.107068
Pielsticker S, Gövert B, Umeki K, Kneer R (2021) Flash pyrolysis kinetics of extracted lignocellulosic biomass components. Front. Energy Res 9:737011. https://doi.org/10.3389/fenrg.2021.737011
Qiao Y, Das O, Zhao SN et al (2020) Pyrolysis kinetic study and reaction mechanism of epoxy glass fiber reinforced plastic by thermogravimetric analyzer (Tg) and tg–ftir (fourier-transform infrared) techniques. Polymers (basel) 12:1–16. https://doi.org/10.3390/polym12112739
De Ras K, Kusenberg M, Vanhove G, et al (2022) A detailed experimental and kinetic modeling study on pyrolysis and oxidation of oxymethylene ether-2 (OME-2). Combust Flame 238. https://doi.org/10.1016/j.combustflame.2021.111914
Rueda-Ordóñez YJ, Arias-Hernández CJ, Manrique-Pinto JF et al (2019) Assessment of the thermal decomposition kinetics of empty fruit bunch, kernel shell and their blend. Bioresour Technol 292:121923. https://doi.org/10.1016/j.biortech.2019.121923
Shahbandeh M (2020) Production volume of palm oil worldwide from 2012/13 to 2019/20. In: Statista. https://www-statista-com.ezproxy.cardiffmet.ac.uk/statistics/613466/olive-oil-production-volume-worldwide/. Accessed 21 Mar 2022
Shrivastava P, Khongphakdi P, Palamanit A et al (2020) Investigation of physicochemical properties of oil palm biomass for evaluating potential of biofuels production via pyrolysis processes. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-019-00596-x
Shuit SH, Tan KT, Lee KT, Kamaruddin AH (2009) Oil palm biomass as a sustainable energy source: a Malaysian case study. Energy 34:1225–1235. https://doi.org/10.1016/j.energy.2009.05.008
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497. https://doi.org/10.1016/j.apenergy.2011.12.056
Sulaiman F, Abdullah N, Gerhauser H, Shariff A (2011) An outlook of Malaysian energy, oil palm industry and its utilization of wastes as useful resources. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2011.06.018
Tian B, Wang X, Zhao W et al (2021) Pyrolysis behaviors, kinetics and gaseous product evolutions of two typical biomass wastes. Catal Today 374:77–85. https://doi.org/10.1016/j.cattod.2020.12.023
Uzun BB, Varol EA, Pütün E (2016) Pyrolysis: a sustainable way from biomass to biofuels and biochar. In: Bruckman VJ, Apaydin Varol E, Uzun BB, Liu J (eds) Biochar. Cambridge University Press, Cambridge, pp 239–265
Vyazovkin S (2001) Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem 22:178–183. https://doi.org/10.1002/1096-987X(20010130)22:23.0.CO;2-%23
Widjaya ER, Chen G, Bowtell L, Hills C (2018) Gasification of non-woody biomass: a literature review. Renew Sustain Energy Rev 89:184–193. https://doi.org/10.1016/j.rser.2018.03.023
**ao R, Yang W, Cong X et al (2020) Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy 201:117537. https://doi.org/10.1016/j.energy.2020.117537
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 45:651–671. https://doi.org/10.1016/S0196-8904(03)00177-8
Yang X, Zhang R, Fu J et al (2014) Pyrolysis kinetic and product analysis of different microalgal biomass by distributed activation energy model and pyrolysis–gas chromatography–mass spectrometry. Bioresour Technol 163:335–342. https://doi.org/10.1016/j.biortech.2014.04.040
Yusoff S (2006) Renewable energy from palm oil – innovation on effective utilization of waste. J Clean Prod 14:87–93. https://doi.org/10.1016/j.jclepro.2004.07.005
Acknowledgements
This research was supported by the Postdoctoral Fellowship from Prince of Songkla University.
Author information
Authors and Affiliations
Contributions
Pranshu Shrivastava: visualization methodology, writing—original draft and investigation. Arkom Palamanit: Conceptualization and supervision. Anil Kumar: writing—review and editing and supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shrivastava, P., Palamanit, A. & Kumar, A. Isoconversional thermal decomposition reaction kinetics of oil palm trunk and rubberwood sawdust for thermochemical conversion processes. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-28998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-28998-5