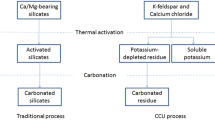
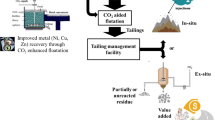
Abstract
Peridotite and serpentinites can be used to sequester CO2 emissions through mineral carbonation. Olivine dissolution rate is directly proportional with temperature, presence of CO2, surface area of mineral particles and presence of ligands and is inversely proportional to pH. Olivine dissolution is better under air flow and increases seven times when rock-inhibiting fungus (Knufia petricola) is used. Olivine dissolution retards as silica layers form during reaction. Sonication, acoustic and concurrent grinding using various grinding medias have been used to artificially break these silica layers and achieve high magnesium extraction. Wet grinding using 50 wt.% ethanol enhanced CO2 uptake of dunite 6.9 times and CO2 uptake of harzburgite by 4.5 times. The best economical process is single-stage concurrent grinding at 130 bar, 185 °C, 15 wt.% solids and 50 wt.% grinding media (zirconia) using 0.64 M NaHCO3. Ratio of grinding media to feed should not be less than 3:1. Yield increases with temperature, pressure, time of reaction, pH and rpm and using additives and grinding media and reducing particle size. This review aims to investigate the progress from 1970s to 2021 on aqueous mineral carbonation of olivine and its naturally available rocks (harzburgite and dunite). This paper comprehensively reviews all aspects of olivine carbonation including olivine dissolution kinetics, effects of grinding and concurrent grinding, thermal activation of olivine feedstock (dunites and harzburgites) as well as chemistry of olivine mineral carbonation. The effects of different reaction parameters on the carbonation yield, role of mineral carbonation accelerators and costs of mineral carbonation process are discussed.










Similar content being viewed by others
Data availability
Data can be provided on demand.
References
Anonymous (2018) Recent global CO2 trend, pp. National Oceanic & Atmospheric Administration Research
Azadi M, Edraki M, Farhang F, Ahn J (2019) Opportunities for mineral carbonation in Australia’s mining industry. Sustainability 11:1250
Baláž P, Turianicová E, Fabián M, Kleiv RA, Briančin J, Obut A (2008) Structural changes in olivine (Mg, Fe)2SiO4 mechanically activated in high-energy mills. Int J Miner Process 88:1–6
Baris K, Ozarslan A, Sahin N (2008) The assesment for CO2 sequestration potential by magnesium silicate minerals in Turkey: cases of Orhaneli-Bursa and Divrigi-Sivas regions. Energy Explor Exploit 26:293–309
Béarat H, McKelvy MJ, Chizmeshya AVG, Sharma R, Carpenter RW (2002) Magnesium hydroxide dehydroxylation/carbonation reaction processes: implications for carbon dioxide mineral sequestration. J Am Ceram Soc 85:742–748
Béarat H, McKelvy MJ, Chizmeshya AVG, Gormley D, Nunez R, Carpenter RW, Squires K, Wolf GH (2006) Carbon sequestration via aqueous olivine mineral carbonation: role of passivating layer formation. Environ Sci Technol 40:4802–4808
Benhelal E 2018: Synthesis and application of mineral carbonation by-products as portland cement substitues University of Newcastle, Australia
Benhelal E, Rashid MI, Holt C, Rayson MS, Brent G, Hook JM, Stockenhuber M, Kennedy EM (2018a) The utilisation of feed and byproducts of mineral carbonation processes as pozzolanic cement replacements. J Clean Prod 186:499–513
Benhelal E, Rashid MI, Rayson M, Brent G, Stockenhuber M, Kennedy E (2018b) Synthesis and characterisation of reactive silica residues from mineral carbonation process. ACEME conference Newcastle, Australia
Benhelal E, Rashid MI, Rayson MS, Prigge J, Molloy TS, Brent GF, Cote A, Stockenhuber M, Kennedy EM (2018c) Study on mineral carbonation of heat activated lizardite at pilot and laboratory scale. J CO2 Utilization 26:230–238
Benhelal E, Rashid MI, Rayson MS, Brent GF, Oliver T, Stockenhuber M, Kennedy EM (2019a) Direct aqueous carbonation of heat activated serpentine: discovery of undesirable side reactions reducing process efficiency. Appl Energy 242:1369–1382
Benhelal E, Rashid MI, Rayson MS, Oliver TK, Brent G, Stockenhuber M, Kennedy EM (2019b) “ACEME”: synthesis and characterization of reactive silica residues from two stage mineral carbonation Process. Environ Prog Sustain Energy 38:e13066
Bodénan F, Bourgeois F, Petiot C, Augé T, Bonfils B, Julcour-Lebigue C, Guyot F, Boukary A, Tremosa J, Lassin A, Gaucher EC, Chiquet P (2014) Ex situ mineral carbonation for CO2 mitigation: evaluation of mining waste resources, aqueous carbonation processability and life cycle assessment (Carmex project). Miner Eng 59:52–63
Brent GF, Petrie JG (2008) CO2 sequestration by mineral carbonation in the Australian context. Eng Australia:1273
Chen Y, Brantley SL (2000) Dissolution of forsteritic olivine at 65°C and 2<pH<5. Chem Geol 165:267–281
Chen ZY, O'Connor WK, Gerdemann SJ (2006) Chemistry of aqueous mineral carbonation for carbon sequestration and explanation of experimental results. Environ Prog 25:161–166
Chipera SJ, Bish DL (2013) Fitting full X-ray diffraction patterns for quantitative analysis: a method for readily quantifying crystalline and disordered phases. Adv Mater Phys Chem 3:47–53
Chizmeshya AVG, McKelvy MJ, Squires K, Carpenter RW, Béarat H 2007 A novel approach to mineral carbonation: enhancing carbonation while avoiding mineral pretreatment process cost, Arizona State University
Crundwell FK (2014) The mechanism of dissolution of forsterite, olivine and minerals of the orthosilicate group. Hydrometallurgy 150:68–82
Dahlin DC, O'Connor WK, Nilsen RP, Rush GE, Walters RP, Turner PC (2000) A method for permanent CO2 mineral carbonation., In:17th Annual International Pittsburgh Coal Conference, DOE/ARC-2000-012., Pittsburgh,PA
Daval D, Sissmann O, Corvisier J, Garcia B, Martinez I, Guyot F, Hellmann R (2010a) The effect of silica coatings on the weathering rates of wollastonite (CaSiO3) and forsterite (Mg 2SiO4): an apparent paradox?, Water-Rock Interaction - Proceedings of the 13th International Conference on Water-Rock Interaction, WRI-13, pp. 713-716
Daval D, Testemale D, Recham N, Tarascon JM, Siebert J, Martinez I, Guyot F (2010b) Fayalite (Fe2SiO4) dissolution kinetics determined by X-ray absorption spectroscopy. Chem Geol 275:161–175
Daval D, Sissmann O, Menguy N, Saldi GD, Guyot F, Martinez I, Corvisier J, Garcia B, Machouk I, Knauss KG, Hellmann R (2011) Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem Geol 284:193–209
Declercq J, Bosc O, Oelkers EH (2013) Do organic ligands affect forsterite dissolution rates? Appl Geochem 39:69–77
Deer WA, Howie RA, Zussman J (1982) Rock-forming minerals: orthosilicates, volume 1A. Geological Society of London
Deer WA, Howie RA, Zussman J (1992) An introduction to the rock-forming minerals (vol. 696). Longman London
Dlugogorski BZ, Balucan RD (2014) Dehydroxylation of serpentine minerals: implications for mineral carbonation. Renew Sust Energ Rev 31:353–367
Dunsmore HE (1992) A geological perspective on global warming and the possibility of carbon dioxide removal as calcium carbonate mineral. Energy Convers Manag 33:565–572
Eikeland E, Blichfeld AB, Tyrsted C, Jensen A, Iversen BB (2015) Optimized carbonation of magnesium silicate mineral for CO2 storage. ACS Appl Mater Interfaces 7:5258–5264
Evans BW, Hattori K, Baronnet A (2013) Serpentinite: what, why, where? Elements 9:99–106
Farhang F, Oliver TK, Rayson M, Brent G, Stockenhuber M, Kennedy E (2016) Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem Eng J 303:439–449
Farhang F, Rayson M, Brent G, Hodgins T, Stockenhuber M, Kennedy E (2017) Insights into the dissolution kinetics of thermally activated serpentine for CO2 sequestration. Chem Eng J 330:1174–1186
Farhang F, Rayson M, Brent G, Stockenhuber M, Kennedy E (2018) Dissolution mechanism of heat activated serpentine and the re-precipitation of silica under constant pH conditions, 6th International Conference on Accelerated Carbonation for Environmental and Material Engineering, ACEME, Newcastle, Australia, pp. pp. 55-64
Farhang F, Oliver TK, Rayson MS, Brent GF, Molloy TS, Stockenhuber M, Kennedy EM (2019) Dissolution of heat activated serpentine for CO2 sequestration: the effect of silica precipitation at different temperature and pH values. J CO2 Utilization 30:123–129
Farhang F, Kennedy E, Stockenhuber M, Brent G, Rayson M (2020) Dehydroxylation of magnesium silicate minerals for carbonation. In: WIPO (Hrsg.)
Gadikota G, Matter J, Kelemen P, Park AHA (2014a) Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3. Phys Chem Chem Phys 16:4679–4693
Gadikota G, Swanson EJ, Zhao H, Park AHA (2014b) Experimental design and data analysis for accurate estimation of reaction kinetics and conversion for carbon mineralization. Ind Eng Chem Res 53:6664–6676
Garcia B, Beaumont V, Perfetti E, Rouchon V, Blanchet D, Oger P, Dromart G, Huc AY, Haeseler F (2010) Experiments and geochemical modelling of CO2 sequestration by olivine: potential, quantification. Appl Geochem 25:1383–1396
Gérardin K, Turri L, Muhr H, Gérard A, Lagadic A, Bertucci S, Lapicque F (2019) Towards viable CO2 sequestration: production of high specific surface area silica by olivine dissolution in concentrated acidic solutions. J Clean Prod 211:1547–1552
Gerdemann SJ, Dahlin CL, O’Conner WK, Penner LR (2003) Carbon dioxide sequestration by aqueous mineral carbonation of magnesium silicate minerals, 2nd annual conference on carbon sequestration, Alexandria, VA
Gerdemann SJ, O'Connor WK, Dahlin DC, Penner LR, Rush H (2007) Ex situ aqueous mineral carbonation. Environ Sci Technol 41:2587–2593
Giammar DE, Bruant RG Jr, Peters CA (2005) Forsterite dissolution and magnesite precipitation at conditions relevant for deep saline aquifer storage and sequestration of carbon dioxide. Chem Geol 217:257–276
Giannoulakis S, Volkart K, Bauer C (2014) Life cycle and cost assessment of mineral carbonation for carbon capture and storage in European power generation. Intl J Greenhouse Gas Control 21:140–157
Goff F, Lackner KS (1998) Carbon dioxide sequestering using uitramaf IC rocks. Environ Geosci 5:89–101
Grandstaff DE (1980) The dissolution of forsteritic olivine from Hawaiian beach sand. Proc. 3rd Intl. Symp. Water-Rock Interaction, pp. 72-74
Grandstaff DE (1986) The dissolution rates of forsteritic olivine from Hawaiian beach sand. Rates of chemical weathering of rocks and minerals, 41-59
Hänchen M, Prigiobbe M, Storti G, Mazzotti M (2006a) Mineral carbonation: kinetic study of olivine dissolution and carbonate precipitation, 8th international conference on greenhouse gas technology, Trondium Norway
Hänchen M, Prigiobbe V, Storti G, Seward TM, Mazzotti M (2006b) Dissolution kinetics of fosteritic olivine at 90–150 °C including effects of the presence of CO2. Geochim Cosmochim Acta 70:4403–4416
Hänchen M, Krevor S, Mazzotti M, Lackner KS (2007) Validation of a population balance model for olivine dissolution. Chem Eng Sci 62:6412–6422
Herzog HJ 1998: CO2 capture, reuse, and sequestration technologies for mitigating global climate change. MIT Laboratory for Energy and the Environment, Cambridge, Massachusetts
Hubbard CR, H. EE (1976): The reference intensity ratio, I/Ic, for computer simulated powder patterns. J Appl Crystallog 9, 169-174
Huijgen WJJ, Ruijg GJ, Comans RNJ, Witkamp GJ (2006) Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind Eng Chem Res 45:9184–9194
Huijgen WJJ, Comans RNJ, Witkamp GJ (2007) Cost evaluation of CO2 sequestration by aqueous mineral carbonation. Energy Convers Manag 48:1923–1935
IEA 2000: (International Energy Agency) CO2 storage as mineral carbonates; prepared by CSMA Consultants Limited Cheltenham, United Kindom
IEA (2014) (International Energy Agency Statistics) 2014 Key world energy statistics
IEA (2021) (Internationa Energy Agency Statistics). World Development Indicators
Jarvis K, Carpenter RW, Windman T, Kim Y, Nunez R, Alawneh F (2009) Reaction mechanisms for enhancing mineral sequestration of CO2. Environ Sci Technol 43:6314–6319
Julcour C, Bourgeois F, Bonfils B, Benhamed I, Guyot F, Bodénan F, Petiot C, Gaucher É (2015) Development of an attrition-leaching hybrid process for direct aqueous mineral carbonation. Chem Eng J 262:716–726
Kelemen P, Shimizu N, Salters VJM (1995) Extraction of mid-ocean-ridge basalt from the upwelling mantle by focused flow of melt in dunite channels. Nature 375:747–753
Khoo HH, Bu J, Wong RL, Kuan SY, Sharratt PN (2011) Carbon capture and utilization: preliminary life cycle CO2, energy, and cost results of potential mineral carbonation. Energy Procedia 4:2494–2501
King HE, Plümper O, Putnis A (2010) Effect of secondary phase formation on the carbonation of olivine. Environ Sci Technol 44:6503–6509
Klein F, Garrido CJ (2011) Thermodynamic constraints on mineral carbonation of serpentinized peridotite. Lithos 126:147–160
Kojima T, Nagamine A, Ueno N, Uemiya S (1997) Absorption and fixation of carbon dioxide by rock weathering. Energy Convers Manag 38:S461–S466
Koukouzas N, Gemeni V, Ziock HJ (2009) Sequestration of CO2 in magnesium silicates, in Western Macedonia, Greece. Int J Miner Process 93:179–186
Lackner KS, Wendt CH, Butt DP, Joyce EL Jr, Sharp DH (1995) Carbon dioxide disposal in carbonate minerals. Energy 20:1153–1170
Lackner KS, Butt DP, Wendt CH (1997) Progress on binding CO2 in mineral substrates. Energy Convers Manag 38(Supplement):S259–S264
Lackner KS (2002) Carbonate chemistry for sequestering fossil carbon. Annu Rev Energy Environ 27:193–232
Lackner KS (2003) A guide to CO2 sequestration. Science 300:1677–1678
Li J, Hitch M (2018) Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner Eng 128:69–83
Li J, Jacobs AD, Hitch M (2019) Direct aqueous carbonation on olivine at a CO2 partial pressure of 6.5 MPa. Energy 173:902–910
Luce RW, Bartlett RW, Parks GA (1972) Dissolution kinetics of magnesium silicates. Geochim Cosmochim Acta 36:35–50
MacKenzie KJD, Meinhold RH (1994) Thermal reactions of chrysotile revisited: a 29Si and 25Mg MAS NMR study. Am Mineral, 43
Maher K, Johnson NC, Jackson A, Lammers LN, Torchinsky AB, Weaver KL, Bird DK, Brown GE (2016) A spatially resolved surface kinetic model for forsterite dissolution. Geochim Cosmochim Acta 174:313–334
Maroto-Valer MM, Fauth DJ, Kuchta ME, Zhang Y, Andrésen JM (2005) Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process Technol 86:1627–1645
Martinez RE, Weber S, Bucher K (2014) Quantifying the kinetics of olivine dissolution in partially closed and closed batch reactor systems. Chem Geol 367:1–12
Merkel TC, Lin H, Wei X, Baker R (2010) Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Membr Sci 359:126–139
Munz IA, Kihle J, Brandvoll Ø, Machenbach I, Carey JW, Haug TA, Johansen H, Eldrup N (2009) A continuous process for manufacture of magnesite and silica from olivine, CO2 and H2O. Energy Procedia 1:4891–4898
O'Connor WK, Dahlin DC, Rush GE, Dahlin CL, Collins WK (2002) Carbon dioxide sequestration by direct mineral carbonation: process mineralogy of feed and products. Miner Metall Process 19:95–101
O’Connor WK, Dahlin DC, Nilsen DN, Gerdemann SJ, Rush GE, Walters RP, Turner PC (2001a) Research status on the seqestration of carbon dioxide by direct aqueous mineral carbonation. 18th Annual International Pittsburgh Coal Conference, Newcastle, NSW, Australia
O’Connor WK, Walters RP, Dahlin DC, Rush GE, Nilsen DN, Turner PC (2001b) Carbon dioxide sequestration by direct aqueous mineral carbonation. Proc. 25th International Technical Conference on Coal Utilization & Fuel Systems, pp. 765
O’Connor WK, Dahlin DC, Rush GE, Gerdemann SJ, Penner LR (2004) Energy and economic considerations for ex situ aqueous mineral carbonation. Proc. 29th International Technical Conference on Coal Utilization & Fuel Systems, pp. 71
O’Connor WK, Dahlin DC, Rush GE, Gerdemann SJ, Penner LR, Nilsen DN 2005 Aqueous mineral carbonation mineral availability, pretreatment, reaction parametrics, and process studies, National Energy Technology Laboratory
Olajire AA (2013) A review of mineral carbonation technology in sequestration of CO2. J Pet Sci Eng 109:364–392
Oliver TK, Dlugogorski BZ, Kennedy EM (2014) Biologically enhanced degassing and precipitation of magnesium carbonates derived from bicarbonate solutions. Miner Eng 61:113–120
Oliver TK, Farhang F, Hodgins TW, Rayson MS, Brent GF, Molloy TS, Stockenhuber M, Kennedy EM (2018) CO2 capture modeling using heat-activated serpentinite slurries. Energy & Fuels
Park AH, Jadhav R, Fan LS (2003) CO2 mineral sequestration: chemically enhanced aqueous carbonation of serpentine. Can J Chem Eng 81:885–890
Park AH, Kelemen P, Matter J, Gadikota G 2012 Geo-chemo-mechanical studies for permanent storage of CO2 in geologic formations, DE-FE0002386. Columbia University, New York, U.S. Department of Energy National Energy Technology Laboratory Carbon Storage R&D Project Review Meeting
Park AHF, L. S (2004) CO2 mineral sequestration: physically activated dissolution of serpentine and pH swing process. Chem Eng Sci 59:5241–5247
Penner LR, O'Connor WK, Dahlin DC, Gerdemann SJ, Rush GE (2004) Mineral carbonation: energy costs of pretreatment options and insights gained from flow loop reaction studies. Exchange Monitor Publications & Forums, Washington, DC
Pokharel R, Gerrits R, Schuessler JA, von Blanckenburg F (2019) Mechanisms of olivine dissolution by rock-inhabiting fungi explored using magnesium stable isotopes. Chem Geol 525:18–27
Pokrovsky OS, Schott J (2000a) Forsterite surface composition in aqueous solutions: a combined potentiometric, electrokinetic, and spectroscopic approach. Geochim Cosmochim Acta 64:3299–3312
Pokrovsky OS, Schott J (2000b) Kinetics and mechanism of forsterite dissolution at 25°C and pH from 1 to 12. Geochim Cosmochim Acta 64:3313–3325
Prigiobbe V, Costa G, Baciocchi R, Hanchen M, Mazzotti M (2009a) The effect of CO2 and salinity on olivine dissolution kinetics at 120 °C. Chem Eng Sci 64:3510–3515
Prigiobbe V, Hänchen M, Costa G, Baciocchi R, Mazzotti M (2009b) Analysis of the effect of temperature, pH, CO2 pressure and salinity on the olivine dissolution kinetics. Energy Procedia 1:4881–4884
Prigiobbe V, Mazzotti M (2011) Dissolution of olivine in the presence of oxalate, citrate, and CO2 at 90 °C and 120 °C. Chem Eng Sci 66:6544–6554
Přikryl J, Jha D, Stefánsson A, Stipp S (2017) Mineral dissolution in porous media: an experimental and modeling study on kinetics, porosity and surface area evolution. Appl Geochem 87:57–70
Přikryl J, Stefánsson A, Pearce CR (2018) Tracing olivine carbonation and serpentinization in CO2-rich fluids via magnesium exchange and isotopic fractionation. Geochim Cosmochim Acta 243:133–148
Rashid MI, Benhelal E, Farhang F, Rayson MS, Brent GF, Stockenhuber M, Kennedy EM (2017) Systematic development of a concurrent grinding technique for application in aqueous mineral carbonation. Chemeca conference, Melbourne, Australia
Rashid MI, Benhelal E, Anderberg L, Farhang F, Oliver TK, Rayson MS, Brent GF, Stockenhuber M, Kennedy EM (2018a) Development of grinding media for aqueous mineral carbonation applications. ACEME conference, Newcastle, Australia
Rashid MI, Benhelal E, Farhang F, Mowla O, Rayson MS, Brent GF, Stockenhuber M, Kennedy EM (2018b) Augmenting the magnesite yield produced during aqueous mineral carbonation of dunite rock. ACEME conference Newcastle, Australia
Rashid MI (2019) Mineral carbonation of CO2 using alternative feedstocks. The University of Newcastle, Australia
Rashid MI, Benhelal E, Farhang F, Oliver TK, Rayson MS, Brent GF, Stockenhuber M, Kennedy EM (2019a) ACEME: direct aqueous mineral carbonation of dunite rock. Environ Prog Sustain Energy 38:e13075
Rashid MI, Benhelal E, Farhang F, Oliver TK, Rayson MS, Brent GF, Stockenhuber M, Kennedy EM (2019b) Development of concurrent grinding for application in aqueous mineral carbonation. J Clean Prod 212:151–161
Rashid MI, Benhelal E, Farhang F, Oliver TK, Stockenhuber M, Kennedy EM (2020a) Application of a concurrent grinding technique for two-stage aqueous mineral carbonation. J CO2 Utilization 42:101347
Rashid MI, Benhelal E, Farhang F, Stockenhuber M, Kennedy EM (2020b) Magnesium leachability of Mg-silicate peridotites: the effect on magnesite yield of a mineral carbonation process. Minerals 10:1091
Rashid MI (2021a) Truth and false-carbon dioxide mitigation technologies. Non Metallic Mater Sci 3:1–5
Rashid MI (2021b) How Ph.D. students start a research. Curr J Appl Sci Technol 40:41–54
Rashid MI, Benhelal E, Farhang F, Oliver TK, Stockenhuber M, Kennedy EM (2021) Application of concurrent grinding in direct aqueous carbonation of magnesium silicates. J CO2 Utilization 48:101516
Rayson M, Brent G, Cote A, Kennedy E, Stockenhuber M, Abu Fara A, Benhelal E, Rashid MI, Anderberg L, Prigge J-D, Molloy TS, Farhang F, Oliver T, Hodgins T, Dawe M (2018) Mineral carbonation for CO2 storage and utilisation: from laboratory to pilot scale. Elizabeth & Frederick White Conference - Frontiers in Gas-Solid Processes from the Atomic Scale to the Parsec, Canberra, Australia
Rigopoulos I, Vasiliades MA, Ioannou I, Efstathiou AM, Godelitsas A, Kyratsi T (2016) Enhancing the rate of ex situ mineral carbonation in dunites via ball milling. Adv Powder Technol 27:360–371
Rigopoulos I, Delimitis A, Ioannou I, Efstathiou AM, Kyratsi T (2018a) Effect of ball milling on the carbon sequestration efficiency of serpentinized peridotites. Miner Eng 120:66–74
Rigopoulos II, Delimitis A, Efstathiou AM, Kyratsi T (2018b) Ball milling effect on the CO2 uptake of mafic and ultramafic rocks: a review. Geosciences 8:406
Rimstidt JD (2015) Diffusion control of quartz and forsterite dissolution rates. Appl Geochem 61:99–108
Sanna A, Uibu M, Caramanna G, Kuusik R, Maroto-Valer MM (2014) A review of mineral carbonation technologies to sequester CO2. Chem Soc Rev 43:8049–8080
Seifritz W (1990) CO2 disposal by means of silicates. Nature 345:486
Shaffer G (2010) Long-term effectiveness and consequences of carbon dioxide sequestration. Nat Geosci 3:464–467
Sipila J, Teir S, Zevenhoven R 2008: Carbon dioxide sequestration by mineral carbonation-Literature review update 2005–2007
Smith RS, Li Z, Dohnálek Z, Kay BD (2014): Adsorption, desorption, and displacement kinetics of H2O and CO2 on forsterite, Mg2SiO4 (011). J Phys Chem C
Summers CA, Dahlin DC, Rush GE, O'Connor WK, Gerdemann SJ (2005) Grinding methods to enhance the reactivity of olivine. Miner Metall Process 22:140–144
Swanson EJ, Fricker KJ, Sun M, Park A-HA (2014) Directed precipitation of hydrated and anhydrous magnesium carbonates for carbon storage. Phys Chem Chem Phys 16:23440–23450
Tans P, Keeling, R. 2010: Trends in atmospheric carbon dioxide: Mauna Loa, Hawaii, National Oceanic and Atmospheric Administration, U.S. Department of Commerce: Washington, DC, 2010
Teir S, Revitzer H, Eloneva S, Fogelholm CJ, Zevenhoven R (2007) Dissolution of natural serpentinite in mineral and organic acids. Int J Miner Process 83:36–46
Walters RP, Chen ZY, Goldberg P, Lackner KS, McKelvy MJ, Ziock H (1999) Mineral carbonation: a viable method for CO2 sequestration. The National Energy Technology Laboratory, Morgantown, West Virginia
Wang F, Dreisinger D, Jarvis M, Hitchins T (2019a) Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner Eng 131:185–197
Wang F, Dreisinger D, Jarvis M, Hitchins T, Dyson D (2019b) Quantifying kinetics of mineralization of carbon dioxide by olivine under moderate conditions. Chem Eng J 360:452–463
Werner M, Hariharan SB, Bortolan AV, Zingaretti D, Baciocchi R, Mazzotti M (2013) Carbonation of activated serpentine for direct flue gas mineralization. Energy Procedia 37:5929–5937
Werner M, Hariharan SB, Mazzotti M (2014): Flue gas CO2 mineralization using thermally activated serpentine: from single- to double-step carbonation. Phys Chem Chem Phys
Wogelius RA, Walther JV (1991) Olivine dissolution at 25°C: effects of pH, CO2, and organic acids. Geochim Cosmochim Acta 55:943–954
Wogelius RA, Walther JV (1992) Olivine dissolution kinetics at near-surface conditions. Chem Geol 97:101–112
Acknowledgements
MIR thanks the University of Newcastle for postgraduate scholarship. We thank Mineral Carbonation International (MCi) for funding this research. MCi has kindly granted us permission to publish this research.
Funding
Mineral Carbonation International provided funds for this research.
Author information
Authors and Affiliations
Contributions
MIR wrote the original draft. EB and FF edited the original draft and provided improvement suggestions. LA wrote the section ‘Petrology and mineralogy of peridotites and serpentinites’. TKO, MR and MS secured the funding and supervised the research. All authors contributed and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 89 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashid, M.I., Benhelal, E., Anderberg, L. et al. Aqueous carbonation of peridotites for carbon utilisation: a critical review. Environ Sci Pollut Res 29, 75161–75183 (2022). https://doi.org/10.1007/s11356-022-23116-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23116-3