Abstract
Immunoglobulin E (IgE)–mediated allergic diseases, including eczema, atopic dermatitis (AD), and allergic rhinitis (AR), have increased prevalence in recent decades. Recent studies have proved that environmental pollution might have correlations with IgE-mediated allergic diseases, but existing research findings were controversial. Thus, we performed a comprehensive meta-analysis from published observational studies to evaluate the risk of long-term and short-term exposure to air pollutants on eczema, AD, and AR in the population (per 10-μg/m3 increase in PM2.5 and PM10; per 1-ppb increase in SO2, NO2, CO, and O3). PubMed, Embase, and Web of Science were searched to identify qualified literatures. The Cochran Q test was used to assess heterogeneity and quantified with the I2 statistic. Pooled effects and the 95% confidence intervals (CIs) were used to evaluate outcome effects. A total of 55 articles were included in the study. The results showed that long-term and short-term exposure to PM10 increased the risk of eczema (PM10, RRlong = 1.583, 95% CI: 1.328, 1.888; RRshort = 1.006, 95% CI: 1.003–1.008) and short-term exposure to NO2 (RRshort = 1.009, 95% CI: 1.008–1.011) was associated with eczema. Short-term exposure to SO2 (RRshort: 1.008, 95% CI: 1.001–1.015) was associated with the risk of AD. For AR, PM2.5 (RRlong = 1.058, 95% CI: 1.014–1.222) was harmful in the long term, and short-term exposure to PM10 (RRshort: 1.028, 95% CI: 1.008–1.049) and NO2 (RRshort: 1.018, 95% CI: 1.007–1.029) were risk factors. The findings indicated that exposure to air pollutants might increase the risk of IgE-mediated allergic diseases. Further studies are warranted to illustrate the potential mechanism for air pollutants and allergic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic diseases are inflammatory disorders that involve various types of cells and factors, including allergens, immunoglobulin (Ig)E, mast cells, basophils, cytokines, and soluble mediators. The etiological mechanisms of allergic diseases are complex (Murrison et al. 2019). The incidence of allergic diseases has increased sharply with increasing industrialization and the accompanying changes to the environment and people’s lifestyles. According to one study conducted by the World Allergy Organization which involved 30 nations/region, approximately 250 million (22%) of the 1.2 billion people in those regions suffered from allergic diseases (Hu et al. 2018). Because of their high prevalence, these diseases pose a serious financial threat to affected households and consume substantial resources in socialized healthcare systems.
Allergic diseases can be divided into two categories, which are IgE mediated and non-IgE mediated. IgE-mediated allergy reactions are typically of rapid onset, and symptoms range from mild to severe. IgE, an antibody class found only in mammals, has unique properties, and plays a central role in the development of acute allergic reactions and IgE-mediated allergic diseases. IgE-mediated allergic diseases involve eczema, atopic dermatitis (AD), and allergic rhinitis (AR). The major risk factors for IgE-mediated allergic diseases studied widely were genetics and immune functions (Renz et al. 2011; Hüls et al. 2019). However, these traditional risk factors were not changed dramatically in recent decades. Therefore, traditional risk factors alone may not be sufficient to explain the massive rise in IgE-mediated allergic disease prevalence.
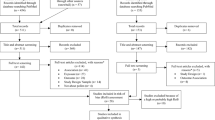
Are long-term or short-term air pollution associated with the development and prevalence of IgE-mediated allergic conditions? Numerous studies have attempted to answer this question, but no consensus has reached. For instance, Schnass et al. conducted a cohort study and concluded that traffic-related air pollution would increase the prevalence of eczema for elders (Schnass et al. 2018). However, Lopez et al. found that long-term air pollution has no adverse effect on adult eczema (Lopez et al. 2021). The same dispute can also be found in AR’s research. Huang et al. reported that the prevalence of AR in children would increase when exposed to PM2.5 (Huang et al. This review was conducted according to the PRISMA framework (Moher et al. 2009) (Table S1). We firstly posed the research question: “Does exposure to air pollutants increase the risks of IgE-mediated allergic diseases?” The included criteria were based on the population, exposure, comparator, and outcomes (PECO) framework. A framework was used to explore the association of air pollutant exposure with health outcomes. P refers to people who have IgE-mediated allergic diseases. E refers to air pollutants. C refers to incremental effect per unit increase in concentration of air pollutants for disease risk. O refers to the outcome of eczema, AR, and AD (Morgan et al. 2018; Marx et al. 2021) (Fig. S1). Embase, PubMed, and Web of Science were searched to find relevant research concerning the association between air pollutants and diseases up to May 2021. Search terms included (“allergic rhinitis” OR allergic rhinitis [MeSH Terms] OR “allergic respiratory diseases” OR allergic respiratory diseases [MeSH Terms] OR “eczema” OR eczema [MeSH Terms] OR “eczematous dermatitides” OR eczematous dermatitides [MeSH Terms] OR “atopic dermatitis” OR atopic dermatitis [MeSH Terms] OR “dermatitis atopic” OR dermatitis atopic [MeSH Terms]) AND (“carbon monoxide” OR “sulfur dioxide” OR “particulate matter” OR “nitrogen dioxide” OR “ozone” OR “PM2.5” OR “PM10”). The detailed search process is shown in supplementary Table S2. The inclusive criteria were as follows: (1) Articles should be epidemiologic studies focusing on the associations between the IgE-mediated allergic diseases with air pollutants exposure. (2) Eczema diagnosis was made according to ICD-10 code L30. ICD-10 code L20 and ICD-9 code 691 were used to classify AD. ICD-10 code J30 and ICD-9 code 477 were principles to detect AR. The classification of these three diseases could also be based on questionnaires of eczema or AD or AR. (3) Studies reported effect estimates (RR, OR, HR, PC) or data that could calculate the effect size. (4) Language was restricted to English. The studies would be excluded were as follows: (1) Animal studies, mechanism studies, reviews and meta-analyses, case reports, treatment effect evaluations, and studies without original data were excluded. (2) Studies focusing on the association between indoor air pollution and prenatal and allergic diseases were also excluded. Endnote software (X9 version) was used to screen eligible literature. All articles were evaluated by two investigators. First, duplicated studies were removed. Then, two investigators (Wang H and Li XB) independently screened remaining studies to select eligible studies. When controversy existed, a third investigator was asked to discuss and resolve the disagreement. For each included study, basic characters were extracted, including disease, first author, publication year, region, study design, sample size, number of cases, age, ICD, data sources of pollutants, term of exposure (short-term or long-term) (Ibrahim et al. 2021), mean concentration of pollutants, and impact effect estimates. Investigators extracted information based on the following principle. A single-pollutant model was used to find the effect of a pollutant, and a multi-pollutant model was utilized to explore the interactions of multiple pollutants on disease risk. If the study contained single-pollutant and multi-pollutant models, the former would be chosen (Yang et al. 2018). Due to inconsistent units of pollutant concentration in some literature, we standardized all effect sizes for every 10-µg/m3 increase in PM2.5 and PM10 and 1-ppb increase in NO2, SO2, CO, and O3 (Fan et al. 2020). The specific formulas were as follows: (1) ppm = 1000 ppb, 1 ppb = M/22.4 (µg/m3). M refers to the molecular weight of each air pollutant. Adjusted relative risk (RR), odds ratio (OR), risk ratio (HR), and percentage change (PC) were used to assess the risk of eczema, AR, and AD (Ning et al. 2021). During data consolidation, PC was transformed into RR. The effect estimates (RR/OR/HR) were standardized. OR and HR were roughly regarded as RR, when outcome events were popular and the effect size was small (Chen et al. 2017). All the effect sizes were pooled by the standardized increment of environmental pollutant concentration. The standardized formulae of effect sizes were as follows: The Newcastle–Ottawa Scale (NOS) and the Office of Health Assessment and Translation (OHAT) tool were used to evaluate the quality of included literature. Among them, NOS was used to assess the reported quality of cohort, case–control, and cross-sectional studies (Lin et al. 2021a). NOS has eight items, and its score ranged from 0 to 9. A study with a score higher than 7 was regarded as a high-quality study. A study with a score of 3 to 6 was intermediate quality. Otherwise, it was low-quality. To the best of our knowledge, there is no effective scale to assess the quality of time-series literature. Therefore, we adopted the quality scale used by Mustafic et al. This scale mainly evaluates three aspects: the validity of the outcome event, the assessment of air pollutant exposure, and the adjustment of confounding factors. When the evaluated document score was 3–5 points, it could be considered high-quality (Mustafic et al. 2012). In a meta-analysis, the OHAT tool was used to assess the risk of bias in each study (Zhang et al. 2021a). Cochran’s Q-test and I2 statistic were used to evaluate the heterogeneity between studies. If I2 is greater than 50%, the heterogeneity is high. Otherwise, the heterogeneity is low. If the p-value of the Q test is less than 0.05, a high heterogeneity is between the studies. Then the random effect model was chosen. The pooled RRs with 95% CIs were estimated using the fixed-effect mode, if the heterogeneity was low. Subgroup analyses were performed by age, region, and study design for each pollutant. Funnel plots were used to represent the publication bias in studies (Bai et al. 2020). In addition, the pooled effect values were tested by determining the age (< 18 years old; ≥ 18 years old; all ages), region, and study design for each pollutant and sensitivity analyses by ICD (Chevalier et al. 2015). Limited by the number of available studies, sensitivity analyses were performed for studies that could be combined in each pollutant. All data analyses were realized by R packages “metafor” and “forestplot” in version 4.0.3. A total of 2478 articles were searched. After screening the titles and abstracts, 150 articles were identified. Finally, fifty-five articles were included thoroughly reading full texts. The process of literature screening is shown in Fig. 1. In four articles, two diseases were simultaneously discussed (Kim et al. 2016; Wang et al. 2016; Min et al. 2020; To et al. 2020). Therefore, seventeen studies were on eczema (six time-series, six cohorts, and five cross-sectional studies), thirty-one studies were on AR (nine time-series, nine cohorts, one case–control, and twelve cross-sectional studies), and eleven studies were on AD (four time-series, five cohorts, and two cross-sectional studies). The information extracted from the literature is shown in Table 1. According to the NOS scale and the OHAT tool, all included studies had high qualities. Scores of articles and details of risk bias assessment were listed as shown in supplementary Table S3 (eczema), Table S4 (AD), and Table S5 (AR). As shown in Table 2 and Fig. S2, the pooled risk for eczema was 1.583 (95% CI: 1.328–1.888) with an increment of 10 μg/m3 in PM10. However, exposures to PM2.5, NO2, and SO2 were not associated with the risk of eczema. The between-study heterogeneity was low than exposure to PM10, NO2, and SO2 (I2 < 50%). The results of publication bias are shown in Fig. S3a-S3b. In subgroup analyses according to age, study design, and region, long-term exposure to PM2.5 and NO2 had no impact on eczema. Other details are shown in Fig. 2a. In addition, due to the limited number of studies on CO and O3, the analysis was not performed. In this meta-analysis, we found that an increment of 10 μg/m3 in PM10 and 1 ppb in NO2 was associated with the risk of eczema (PM10, RR = 1.006, 95% CI: 1.003–1.008; NO2: RR = 1.009, 95% CI: 1.008–1.011), and PM2.5 and SO2 were irrelevant with the risk of eczema (Table 2; Fig. S2). The between-study heterogeneity and publication bias are illustrated in Table 2 and Fig. S3c-S3f. Sensitivity analyses by ICD did not change the overall effect in PM10 and NO2 (Fig. S4). In all age groups, PM10 and NO2 increased the risk of eczema. The study design (cross-sectional) group showed no correlation on eczema in PM2.5, PM10, and NO2, and the study design (time-series) group suggested that each increment unit in PM2.5, PM10, and NO2 increased the risk of eczema. Exposures to PM2.5, PM10, and NO2 were related to the risk of eczema in region (Asia) group (Fig. 2a) Studies on pollutants CO and O3 were not enough for combining analyses at present. A total of eleven studies on AD were included in this meta-analysis. However, significant associations were not found between AD and exposure to six air pollutants (Table 2; Fig. S5). The results of heterogeneities and funnel plots are displayed in Table 2 and Fig. S6a-S6f. From Fig. 2b, ICD sensitivity analyses did not change the overall estimates (Fig. S4). The age under 18 group indicated that each increment unit in PM10 and CO was harmful to the occurrence of AD with low between-study heterogeneity (I2 < 50%), while PM2.5, NO2, SO2, and O3 were irrelevant. The study design (cohort) group was observed to have a significant association between AD and SO2 (Fig. 2b). The evidence of the detailed subgroup analyses is showed in Fig. 2b. As we can see from Table 2 and Fig. S5, SO2 increased the risk of AD by 1.008 with an increment of 1 ppb (95% CI: 1.001–1.015). PM10, NO2, and O3 had no impact on AD. The sensitivity analyses of ICD suggested that PM10, SO2, and O3 were correlated with AD in short time (Fig. S4). PM10 increased the risk of AD in all age groups. The details in subgroup analyses are shown in Fig. 2b. The results showed that only PM2.5 (an increment of 10 μg/m3) had a harmful effect to the occurrence of AR (RR = 1.058, 95% CI: 1.014–1.222), and the heterogeneity between articles was high (I2 > 50%) (Table 2; Fig. S7). PM10, NO2, SO2, CO, and O3 had no effect on the risk of AR, and the specific analyses are shown in Fig. S7. The evidence of publication bias is displayed in supplementary material Fig. S8a-S8f. Sensitivity analyses in the ICD group showed no association between AR and PM2.5 and O3 (Fig. S4). According to subgroup analyses in Fig. 2c, in the age under 18 group, the effect estimate was increased by 1.133 for an increment of 10 μg/m3 of PM2.5. The study design (cross-sectional) group found a correlation between PM10 and AR. In the region (Asia) group, PM2.5 strengthened the risk of AR, and all results were as shown in Fig. 2c. As shown in Table 2 and Fig. S7, an increment unit in PM10 and NO2 was associated with the risk of AR (PM10: RR = 1.028, 95% CI: 1.008–1.049; NO2: RR = 1.018, 95% CI: 1.007–1.029). PM2.5, SO2, CO, and O3 were not associated with the risk of AR. High heterogeneity was observed in PM2.5, PM10, NO2, SO2, and O3 (I2 > 50%). Funnel plots for publication bias are displayed in Fig. S8g-S8l. Sensitivity analyses by ICD, PM10, NO2, and SO2 were harmful factors for the risk of AR. In a subgroup analysis of age, PM10 was harmful to the population aged < 18. For all age groups, PM2.5, PM10, NO2, and SO2 increased the risk of AR. The study design (time-series) group indicated that PM10 and NO2 were potential risk factors for AR. In the region (Asia) group, PM10 and NO2 were associated with AR risk, and more details are shown in Fig. 2c. In the current systematic review and meta-analysis of 55 epidemiological studies, we performed a comprehensive evaluation of available data on ambient air pollution and IgE-mediated allergic diseases. Most included studies reported a positive association between certain air pollutants’ level and greater risk of IgE-mediated allergic diseases (Table 1). The meta-analysis results showed significant associations of long-term exposure to PM2.5 with AR and AD. Besides, long-term exposure to PM10 was found to be related to the increased risk of eczema. Although point estimates indicate higher risk of exposure to NO2, SO2, CO, and O3, the difference was not statistically significant in terms of confidence intervals. Short-term exposures to PM10 and NO2 were related with eczema and AR, and short-term exposures to SO2 and PM2.5 were associated with AD. Actually, it is difficult to assess the health effects of individual ambient pollutants, because these substances are rarely produced in isolation. For example, a study of 317,926 children found a significant positive association between traffic-related pollution and AD in both sexes. However, analysis of individual traffic-related pollutants only revealed associations of AD with NOx and CO in females (Lee et al. 2008). Therefore, synergistic effects of multiple pollutants can be missed when studying the effects of a single pollutant. Besides, between-study heterogeneity for these meta-analyses was high. These might be partly explained by varied study designs, regions, ages outcome definition, and exposure assessment of the included studies. Results of studies highlighted that early childhood exposure to air pollutants from birth to 5 years of age was associated with new onset of IgE-mediated allergic diseases throughout childhood and there was evidence to suggest that air pollutants may have an ongoing effect with a lag time of about 3 years (Bowatte et al. 2015). Therefore, the longer the observational duration from birth, the higher the likelihood of finding the relationship between air pollutants and IgE-mediated allergic diseases. When we conducted subgroup analyses based on age, we found that people age under 18 had a higher risk of IgE-mediated allergic diseases compared with adults aged above 18. Nevertheless, many of these subgroups are I2 > 50, so age might not be the only source of heterogeneity. Rapid urbanization, economic growth, increase in the number of vehicles, clean energy use, and proportion of primary and secondary industries reveal the different kinds and levels of pollutants in different regions (Song et al. Not applicable. Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M, Esplugues A, Fernández-Somoano A, Lertxundi A, Tardón A, Sunyer J (2013) Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect 121:387–392. https://doi.org/10.1289/ehp.1205281 Anderson HR, Ruggles R, Pandey KD, Kapetanakis V, Brunekreef B, Lai CK, Strachan DP, Weiland SK, ISAAC Phase One Study Group (2010) Ambient particulate pollution and the world-wide prevalence of asthma, rhinoconjunctivitis and eczema in children: Phase One of the International Study of Asthma and Allergies in Childhood (ISAAC). Occup Environ Med 67:293–300. https://doi.org/10.1136/oem.2009.048785 Arnedo-Pena A, García-Marcos L, CarvajalUrueña I, Busquets Monge R, Morales Suárez-Varela M, Miner Canflanca I, BatllesGarrido J, Blanco Quirós A, López-Silvarrey Varela A, García Hernández G, AguinagaOntoso I, González Díaz C (2009) Contaminación del aire y síntomas recientes de asma, rinitis alérgica y eccema atópico en escolares de 6 y 7 años [Air pollution and recent symptoms of asthma, allergic rhinitis, and atopic eczema in schoolchildren aged between 6 and 7 years]. Arch Bronconeumol 45:224–229. https://doi.org/10.1016/j.arbres.2008.10.004 Baek JO, Cho J, Roh JY (2021) Associations between ambient air pollution and medical care visits for atopic dermatitis. Environ Res 195:110153. https://doi.org/10.1016/j.envres.2020.110153 Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, Duan R, Duan H, Kou C, Li Y, Sun Z (2020) Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res 185:109471. https://doi.org/10.1016/j.envres.2020.109471 Belugina IN, Yagovdik NZ, Belugina OS, Belugin SN (2018) Outdoor environment, ozone, radionuclide-associated aerosols and incidences of infantile eczema in Minsk, Belarus. J Eur Acad Dermatol Venereol 32:1977–1985. https://doi.org/10.1111/jdv.15063 Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC (2015) The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 70:245–256. https://doi.org/10.1111/all.12561 Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B (2007) Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 29:879–888. https://doi.org/10.1183/09031936.00083406 Burte E, Leynaert B, Bono R, Brunekreef B, Bousquet J, Carsin AE, De Hoogh K, Forsberg B, Gormand F, Heinrich J, Just J, Marcon A, Künzli N, Nieuwenhuijsen M, Pin I, Stempfelet M, Sunyer J, Villani S, Siroux V, Jarvis D, Jacquemin B (2018) Association between air pollution and rhinitis incidence in two European cohorts. Environ Int 115:257–266. https://doi.org/10.1016/j.envint.2018.03.021 Cachon BF, Firmin S, Verdin A, Ayi-Fanou L, Billet S, Cazier F, Martin PJ, Aissi F, Courcot D, Sanni A, Shirali P (2014) Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM(2.5) and PM(>2.5)) collected from Cotonou, Benin. Environ Pollut 185:340–351. https://doi.org/10.1016/j.envpol.2013.10.026 Chen CC, Chiu HF, Yang CY (2016a) Air pollution exposure and daily clinical visits for allergic rhinitis in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A 79:494–501. https://doi.org/10.1080/15287394.2016.1182002 Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, Deng Q, Huang C, Hu Y, Zou Z, Liu W, Wang J, Lu C, Qian H, Yang X, Zhang X, Qu F, Sundell J, Zhang Y, Li B, Zhao Z (2018) The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ Pollut 232:329–337. https://doi.org/10.1016/j.envpol.2017.08.072 Chen J, Peng L, He S, Li Y, Mu Z (2016b) Association between environmental factors and hospital visits among allergic patients: a retrospective study. Asian Pac J Allergy Immunol 34:21–9. https://doi.org/10.12932/AP0639.34.1.2016 Chen JP, Chen GC, Wang XP, Qin L, Bai Y (2017) Dietary fiber and metabolic syndrome: a meta-analysis and review of related mechanisms. Nutrients 10:24. https://doi.org/10.3390/nu10010024 Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanović A, Hagemann S, Montet X, Seimbille Y, Zamboni N, Hapfelmeier S, Trajkovski M (2015) Gut microbiota orchestrates energy homeostasis during cold. Cell 163:1360–1374. https://doi.org/10.1016/j.cell.2015.11.004 Chu H, **n J, Yuan Q, Wang M, Cheng L, Zhang Z, Lu M (2019) The effects of particulate matters on allergic rhinitis in Nan**g, China. Environ Sci Pollut Res Int 26:11452–11457. https://doi.org/10.1007/s11356-019-04593-5 Chung HY, Hsieh CJ, Tseng CC, Yiin LM (2016) Association between the first occurrence of allergic rhinitis in preschool children and air pollution in Taiwan. Int J Environ Res Public Health 13:268. https://doi.org/10.3390/ijerph13030268 de Marco R, Poli A, Ferrari M, Accordini S, Giammanco G, Bugiani M, Villani S, Ponzio M, Bono R, Carrozzi L, Cavallini R, Cazzoletti L, Dallari R, Ginesu F, Lauriola P, Mandrioli P, Perfetti L, Pignato S, Pirina P, Struzzo P, ISAYA study group Italian Study on Asthma in Young Adults (2002) The impact of climate and traffic-related NO2 on the prevalence of asthma and allergic rhinitis in Italy. Clin Exp Allergy 32:1405–1412. https://doi.org/10.1046/j.1365-2745.2002.01466.x Deng S, Huang D, Wang W, Yan H, Li S, **ang H (2019) Associations of gestational and the first year of life exposure to ambient air pollution with childhood eczema in Hubei, China. Environ Sci Pollut Res Int 26:23842–23849. https://doi.org/10.1007/s11356-019-05633-w Fan SJ, Heinrich J, Bloom MS, Zhao TY, Shi TX, Feng WR, Sun Y, Shen JC, Yang ZC, Yang BY, Dong GH (2020) Ambient air pollution and depression: a systematic review with meta-analysis up to 2019. Sci Total Environ 701:134721. https://doi.org/10.1016/j.scitotenv.2019.134721 Fuertes E, Brauer M, MacIntyre E, Bauer M, Bellander T, von Berg A, Berdel D, Brunekreef B, Chan-Yeung M, Gehring U, Herbarth O, Hoffmann B, Kerkhof M, Klümper C, Koletzko S, Kozyrskyj A, Kull I, Heinrich J, Melén E, Pershagen G, TAG Study Group (2013a) Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG Study. J Allergy Clin Immunol 132:342–52.e2. https://doi.org/10.1016/j.jaci.2013.03.007 Fuertes E, Standl M, Cyrys J, Berdel D, von Berg A, Bauer CP, Krämer U, Sugiri D, Lehmann I, Koletzko S, Carlsten C, Brauer M, Heinrich J (2013b) A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ 1:e193. https://doi.org/10.7717/peerj.193 Fuertes E, Sunyer J, Gehring U, Porta D, Forastiere F, Cesaroni G, Vrijheid M, Guxens M, Annesi-Maesano I, Slama R, Maier D, Kogevinas M, Bousquet J, Chatzi L, Lertxundi A, Basterrechea M, Esplugues A, Ferrero A, Wright J, Mason D, Jacquemin B (2020) Associations between air pollution and pediatric eczema, rhinoconjunctivitis and asthma: a meta-analysis of European birth cohorts. Environ Int 136:105474. https://doi.org/10.1016/j.envint.2020.105474 Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B (2010) Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med 181:596–603. https://doi.org/10.1164/rccm.200906-0858OC Guo Q, Liang F, Tian L, Schikowski T, Liu W, Pan X (2019) Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Bei**g: a time-stratified case-crossover analysis. Environ Sci Process Impacts 21:163–173. https://doi.org/10.1039/c8em00494c Hao S, Yuan F, Pang P, Yang B, Jiang X, Yan A (2021) Early childhood traffic-related air pollution and risk of allergic rhinitis at 2–4 years of age modification by family stress and male gender: a case-control study in Shenyang, China. Environ Health Prev Med 26:48. https://doi.org/10.1186/s12199-021-00969-7 Hu J, Chen J, Ye L, Cai Z, Sun J, Ji K (2018) Anti-IgE therapy for IgE-mediated allergic diseases: from neutralizing IgE antibodies to eliminating IgE(+) B cells. Clin Transl Allergy 8:27. https://doi.org/10.1186/s13601-018-0213-z Hu Y, Xu Z, Jiang F, Li S, Liu S, Wu M, Yan C, Tan J, Yu G, Hu Y, Yin Y, Tong S (2020) Relative impact of meteorological factors and air pollutants on childhood allergic diseases in Shanghai, China. Sci Total Environ 706:135975. https://doi.org/10.1016/j.scitotenv.2019.135975 Huang Q, Ren Y, Liu Y, Liu S, Liu F, Li X, Li B, Hou Y, Lu Y, Li S, Yan H, **ang H (2019) Associations of gestational and early life exposure to air pollution with childhood allergic rhinitis. Atmos Environ 200:190–196. https://doi.org/10.1016/j.atmosenv.2018.11.055 Hüls A, Klümper C, MacIntyre EA, Brauer M, Melén E, Bauer M, Berdel D, Bergström A, Brunekreef B, Chan-Yeung M, Fuertes E, Gehring U, Gref A, Heinrich J, Standl M, Lehmann I, Kerkhof M, Koppelman GH, Kozyrskyj AL, Pershagen G, TAG Study Group (2018) Atopic dermatitis: interaction between genetic variants of GSTP1, TNF, TLR2, and TLR4 and air pollution in early life. Pediatr Allergy Immunol 29:596–605. https://doi.org/10.1111/pai.12903 Hüls A, Abramson MJ, Sugiri D, Fuks K, Krämer U, Krutmann J, Schikowski T (2019) Nonatopic eczema in elderly women: effect of air pollution and genes. J Allergy Clin Immunol 143:378-385.e9. https://doi.org/10.1016/j.jaci.2018.09.031 Hwang BF, Jaakkola JJ, Lee YL, Lin YC, Guo YL (2006) Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir Res 7:23. https://doi.org/10.1186/1465-9921-7-23 Ibrahim MF, Hod R, Nawi AM, Sahani M (2021) Association between ambient air pollution and childhood respiratory diseases in low- and middle-income Asian countries: a systematic review. Atmos Environ 256:118422. https://doi.org/10.1016/j.atmosenv.2021.118422 Jo EJ, Lee WS, Jo HY, Kim CH, Eom JS, Mok JH, Kim MH, Lee K, Kim KU, Lee MK, Park HK (2017) Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respir Med 124:79–87. https://doi.org/10.1016/j.rmed.2017.02.010 Karagün E, Yıldız P, Cangür Ş (2021) Effects of climate and air pollution factors on outpatient visits for eczema: a time series analysis. Arch Dermatol Res 313:49–55. https://doi.org/10.1007/s00403-020-02115-9 Kathuria P, Silverberg JI (2016) Association of pollution and climate with atopic eczema in US children. Pediatr Allergy Immunol 27:478–485. https://doi.org/10.1111/pai.12543 Kim BJ, Kwon JW, Seo JH, Kim HB, Lee SY, Park KS, Yu J, Kim HC, Leem JH, Sakong J, Kim SY, Lee CG, Kang DM, Ha M, Hong YC, Kwon HJ, Hong SJ (2011) Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol 107:214–9.e1. https://doi.org/10.1016/j.anai.2011.05.025 Kim J, Han Y, Seo SC, Lee JY, Choi J, Kim KH, Woo SY, Kim EH, Kwon HJ, Cheong HK, Oh I, Ahn K (2016) Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc 37:e1–e7. https://doi.org/10.2500/aap.2016.37.3918 Kim SH, Lee J, Oh I, Oh Y, Sim CS, Bang JH, Park J, Kim Y (2021) Allergic rhinitis is associated with atmospheric SO2: follow-up study of children from elementary schools in Ulsan, Korea. PLoS One 16:e0248624. https://doi.org/10.1371/journal.pone.0248624 Kim YM, Kim J, Han Y, Jeon BH, Cheong HK, Ahn K (2017) Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS ONE 12:e0175229. https://doi.org/10.1371/journal.pone.0175229 Krämer U, Sugiri D, Ranft U, Krutmann J, von Berg A, Berdel D, Behrendt H, Kuhlbusch T, Hochadel M, Wichmann HE, Heinrich J, GINIplus and LISAplus study groups, (2009) Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J Dermatol Sci 56(2):99–105. https://doi.org/10.1016/j.jdermsci.2009.07.014 Lee YL, Su HJ, Sheu HM, Yu HS, Guo YL (2008) Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J Invest Dermatol 128:2412–2420. https://doi.org/10.1038/jid.2008.110 Lee JT, Cho YS, Son JY (2010) Relationship between ambient ozone concentrations and daily hospital admissions for childhood asthma/atopic dermatitis in two cities of Korea during 2004–2005. Int J Environ Health Res 20:1–11. https://doi.org/10.1080/09603120903254033 Lawal AO (2017) Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol Lett 270:88–95. https://doi.org/10.1016/j.toxlet.2017.01.017 Li A, Fan L, **e L, Ren Y, Li L (2018) Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis. J Eur Acad Dermatol Venereol 32:486–494. https://doi.org/10.1111/jdv.1473 Li Q, Yang Y, Chen R, Kan H, Song W, Tan J, Xu F, Xu J (2016) Ambient air pollution, meteorological factors and outpatient visits for eczema in Shanghai, China: a time-series analysis. Int J Environ Res Public Health 1313:1106. https://doi.org/10.3390/ijerph13111106 Lin L, Li T, Sun M, Liang Q, Ma Y, Wang F, Duan J, Sun Z (2021a) Effect of particulate matter exposure on the prevalence of allergic rhinitis in children: a systematic review and meta-analysis. Chemosphere 268:128841. https://doi.org/10.1016/j.chemosphere.2020.128841 Lin YT, Shih H, Jung CR, Wang CM, Chang YC, Hsieh CY, Hwang BF (2021b) Effect of exposure to fine particulate matter during pregnancy and infancy on paediatric allergic rhinitis. Thorax 76:568–574. https://doi.org/10.1136/thoraxjnl-2020-215025 Liu K, Li S, Qian ZM, Dharmage SC, Bloom MS, Heinrich J, Jalaludin B, Markevych I, Morawska L, Knibbs LD, Hinyard L, **an H, Liu S, Lin S, Leskinen A, Komppula M, Jalava P, Roponen M, Hu LW, Zeng XW, Dong GH (2020) Benefits of influenza vaccination on the associations between ambient air pollution and allergic respiratory diseases in children and adolescents: New insights from the Seven Northeastern Cities study in China. Environ Pollut 256:113434. https://doi.org/10.1016/j.envpol.2019.113434 Liu W, Cai J, Huang C, Hu Y, Fu Q, Zou Z, Sun C, Shen L, Wang X, Pan J, Huang Y, Chang J, Zhao Z, Sun Y, Sundell J (2016) Associations of gestational and early life exposures to ambient air pollution with childhood atopic eczema in Shanghai, China. Sci Total Environ 572:34–42. https://doi.org/10.1016/j.scitotenv.2016.07.197 Li S, Wu W, Wang G, Zhang X, Guo Q, Wang B, Cao S, Yan M, Pan X, Xue T, Gong J, Duan X (2022) Association between exposure to air pollution and risk of allergic rhinitis: a systematic review and meta-analysis. Environ Res 205:112472. https://doi.org/10.1016/j.envres.2021.112472 Lopez DJ, Lodge CJ, Bui DS, Waidyatillake NT, Su JC, Perret JL, Knibbs LD, Erbas B, Thomas PS, Hamilton GS, Thompson BR, Abramson MJ, Walters EH, Dharmage SC, Bowatte G, Lowe AJ (2021) Association between ambient air pollution and development and persistence of atopic and non-atopic eczema in a cohort of adults. Allergy 76(8):2524–2534. https://doi.org/10.1111/all.14783 Lu C, Deng QH, Ou CY, Liu WW, Sundell J (2013) Effects of ambient air pollution on allergic rhinitis among preschool children in Changsha, China. Chin Sci Bull 58:4252–4258 Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097 Martin RA, Ather JL, Daggett R, Hoyt L, Alcorn JF, Suratt BT, Weiss DJ, Lundblad LK, Poynter ME (2013) The endogenous Th17 response in NO2-promoted allergic airway disease is dispensable for airway hyperresponsiveness and distinct from Th17 adoptive transfer. PLoS ONE 8:e74730. https://doi.org/10.1371/journal.pone.0074730 Marx T, Bernard N, Kepka S, Gérazime A, Mauny F, Desmettre T (2021) Pneumothorax and the environment: a systematic review of the impact of air pollution and meteorology, and a meta-analysis on meteorology factors. Environ Pollut 283:117089. https://doi.org/10.1016/j.envpol.2021.117089 Min KD, Yi SJ, Kim HC, Leem JH, Kwon HJ, Hong S, Kim KS, Kim SY (2020) Association between exposure to traffic-related air pollution and pediatric allergic diseases based on modeled air pollution concentrations and traffic measures in Seoul, Korea: a comparative analysis. Environ Health 19:6. https://doi.org/10.1186/s12940-020-0563-6 Morgan RL, Whaley P, Thayer KA, Schünemann HJ (2018) Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 121:1027–1031. https://doi.org/10.1016/j.envint.2018.07.015 Murrison LB, Brandt EB, Myers JB, Hershey GKK (2019) Environmental exposures and mechanisms in allergy and asthma development. J Clin Investig 129:1504–1515. https://doi.org/10.1172/JCI124612 Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer MC, Marijon E, Vernerey D, Empana JP, Jouven X (2012) Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307:713–721. https://doi.org/10.1001/jama.2012.126 Ngoc L, Park D, Lee Y, Lee YC (2017) Systematic review and meta-analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health 14:1458. https://doi.org/10.3390/ijerph14121458 Ning J, Zhang Y, Hu H, Hu W, Li L, Pang Y, Ma S, Niu Y, Zhang R (2021) Association between ambient particulate matter exposure and metabolic syndrome risk: a systematic review and meta-analysis. Sci Total Environ 782:146855. https://doi.org/10.1016/j.scitotenv.2021.146855 Park SK, Kim JS, Seo HM (2021) Exposure to air pollution and incidence of atopic dermatitis in the general population: a national population-based retrospective cohort study. J Am Acad Dermatol Adv. Online Publication. https://doi.org/10.1016/j.jaad.2021.05.061 Renz H, Conrad M, Brand S, Teich R, Garn H, Pfefferle PI (2011) Allergic diseases, gene-environment interactions. Allergy 66 Suppl 95:10–12. https://doi.org/10.1111/j.1398-9995.2011.02622.x Song C, Wu L, **e Y, He J, Chen X, Wang T, Lin Y, ** T, Wang A, Liu Y, Dai Q, Liu B, Wang YN, Mao H (2017) Air pollution in China: status and spatiotemporal variations. Environ Pollut 227:334–347. https://doi.org/10.1016/j.envpol.2017.04.075 Schnass W, Hüls A, Vierkötter A, Krämer U, Krutmann J, Schikowski T (2018) Traffic-related air pollution and eczema in the elderly: findings from the SALIA cohort. Int J Hyg Environ Health 221:861–867. https://doi.org/10.1016/j.ijheh.2018.06.002 Tang KT, Ku KC, Chen DY, Lin CH, Tsuang BJ, Chen YH (2017) Adult atopic dermatitis and exposure to air pollutants-a nationwide population-based study. Ann Allergy Asthma Immunol 118:351–355. https://doi.org/10.1016/j.anai.2016.12.005 Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N (2008) Particulate matter (PM(2.5), PM(10–2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A 71:512–520. https://doi.org/10.1080/15287390801907459 Teng B, Zhang X, Yi C, Zhang Y, Ye S, Wang Y, Tong DQ, Lu B (2017) The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, Northeastern China. Int J Environ Res Public Health 14:226. https://doi.org/10.3390/ijerph14030226 To T, Zhu J, Stieb D, Gray N, Fong I, Pinault L, Jerrett M, Robichaud A, Ménard R, van Donkelaar A, Martin RV, Hystad P, Brook JR, Dell S (2020) Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur Respir J 55:1900913. https://doi.org/10.1183/13993003.00913-2019 Tan X, Han L, Zhang X, Zhou W, Li W, Qian Y (2021) A review of current air quality indexes and improvements under the multi-contaminant air pollution exposure. J Environ Manage 279:111681. https://doi.org/10.1016/j.jenvman.2020.111681 Wang IJ, Tung TH, Tang CS, Zhao ZH (2016) Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health 219:66–71. https://doi.org/10.1016/j.ijheh.2015.09.001 Wang J, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Deng Q, Lu C, Qian H, Yang X, Sun Y, Sundell J, Norbäck D (2021) Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci Total Environ 751:141734. https://doi.org/10.1016/j.scitotenv.2020.141734 Wang M, Wang S, Wang X, Tian Y, Wu Y, Cao Y, Song J, Wu T, Hu Y (2020) The association between PM2.5 exposure and daily outpatient visits for allergic rhinitis: evidence from a seriously air-polluted environment. Int J Biometeorol 64:139–144. https://doi.org/10.1007/s00484-019-01804-z Wang XW, Tian YH, Cao YY, Song J, Li M, Wu Y, Wang MY, Huang Z, Hu YH (2019) Association between fine particulate air pollution and outpatient visits for eczema in Bei**g, China: a time-series analysis. Biomed Environ Sci 32:624–627. https://doi.org/10.3967/bes2019.080 Wood HE, Marlin N, Mudway IS, Bremner SA, Cross L, Dundas I, Grieve A, Grigg J, Jamaludin JB, Kelly FJ, Lee T, Sheikh A, Walton R, Griffiths CJ (2015) Effects of air pollution and the introduction of the London Low Emission Zone on the prevalence of respiratory and allergic symptoms in schoolchildren in East London: a sequential cross-sectional study. PLoS ONE 10:e0109121. https://doi.org/10.1371/journal.pone.0109121 Yang L, Hou XY, Wei Y, Thai P, Chai F (2017) Biomarkers of the health outcomes associated with ambient particulate matter exposure. Sci Total Environ 579:1446–1459. https://doi.org/10.1016/j.scitotenv.2016.11.146 Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, Dong GH (2018) Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut 235:576–588. https://doi.org/10.1016/j.envpol.2018.01.001 Zhang F, Wang W, Lv J, Krafft T, Xu J (2011) Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Bei**g, China. Sci Total Environ 409:2486–2492. https://doi.org/10.1016/j.scitotenv.2011.04.007 Zhang H, Zhang X, Wang Q, Xu Y, Feng Y, Yu Z, Huang C (2021a) Ambient air pollution and stillbirth: an updated systematic review and meta-analysis of epidemiological studies. Environ Pollut 278:116752. https://doi.org/10.1016/j.envpol.2021.116752 Zhang L, **g D, Lu Q, Shen S (2021b) NO(2) exposure increases eczema outpatient visits in Guangzhou, China: an indication for hospital management. BMC Public Health 21:506. https://doi.org/10.1186/s12889-021-10549-7 Zhou PE, Qian ZM, McMillin SE, Vaughn MG, **e ZY, Xu YJ, Lin LZ, Hu LW, Yang BY, Zeng XW, Zhang WJ, Liu RQ, Chen G, Dong GH (2021) Relationships between long-term ozone exposure and allergic rhinitis and bronchitic symptoms in Chinese children. Toxics 9:221. https://doi.org/10.3390/toxics9090221 Zou QY, Shen Y, Ke X, Hong SL, Kang HY (2018) Exposure to air pollution and risk of prevalence of childhood allergic rhinitis: A meta-analysis. Int J Pediatr Otorhinolaryngol 112:82–90. https://doi.org/10.1016/j.ijporl.2018.06.039 We thank the participants for joining our study and reviewers for their valuable suggestion. This study was supported by the National Natural Science Foundation of China (81803310); Undergraduate Innovation and Entrepreneurship Training Program in Anhui Province (S201910366064); Emergency Research Project of Novel Coronavirus Infection of Anhui Medical University (YJGG202003); the Grants for Scientific Research of BSKY (XJ201619) from Anhui Medical University; and Research Fund of Anhui Institute of Translational Medicine (2021zhyx-C21). Hua Wang: performed the data analysis, writing—original draft. **an-Bao Li: performed the data analysis, writing—original draft. **u-Jie Chu: investigation, data curation. Nv-Wei Cao: formal analysis, helped revise the manuscript. Hong Wu: investigation. Rong-Gui Huang: investigation. Bao-Zhu Li (lbz88730@163.com) and Dong-Qing Ye (anhuiydq@126.com): conceptualization, project administration, funding acquisition, writing—original draft. Not applicable. Not applicable (this study does not contain any individual person’s data in any form). The authors declare that they agree with the publication of this paper in this journal. The authors declare no competing interests. Responsible Editor: Lotfi Aleya Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Below is the link to the electronic supplementary material. Fig. S1 Research method conceptual framework diagram (PNG 497 kb) Fig. S2 Forest plot of included studies of air pollutants exposure and eczema (PNG 676 kb) Fig. S3 Funnel plot of studies on the effect of air pollutants on eczema (PNG 484 kb) Fig. S4 Sensitivity analyses by ICD of IgE mediated allergic diseases (PNG 1299 kb) Fig. S5 Forest plot of included studies of air pollutants exposure and AD (PNG 826 kb) Fig. S6 Funnel plot of studies on the effect of air pollutants on AD (long-term) (PNG 492 kb) Fig. S7 Forest plot of included studies of air pollutants exposure and AR (PNG 2260 kb) Fig. S8 Funnel plot of studies on the effect of air pollutants on AR (PNG 618 kb) Wang, H., Li, XB., Chu, XJ. et al. Ambient air pollutants increase the risk of immunoglobulin E–mediated allergic diseases: a systematic review and meta-analysis.
Environ Sci Pollut Res 29, 49534–49552 (2022). https://doi.org/10.1007/s11356-022-20447-z Received: Accepted: Published: Issue Date: DOI: https://doi.org/10.1007/s11356-022-20447-zMaterial and methods
Search strategy
Inclusion and exclusion criteria
Data extraction
Data synthesis
Quality and risk bias assessment
Statistical analysis
Results
Characteristics of included studies
Relationship between air pollution and eczema
Effect of long-term air pollution exposure on eczema
Effect of short-term air pollution exposure on eczema
Relationship between air pollution and AD
Effect of long-term air pollution exposure on AD
Effect of short-term air pollution exposure on AD
Relationship between air pollution and AR
Effect of long-term air pollution exposure on AR
Effect of short-term air pollution exposure on AR
Discussion
Data availability
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding author
Ethics declarations
Ethics approval
Consent to participate
Consent for publication
Competing interests
Additional information
Publisher's note
Supplementary Information

ESM 2

ESM 3

ESM 4

ESM 5

ESM 6

ESM 7

ESM 8

ESM 9
Rights and permissions
About this article
Cite this article
Keywords