Abstract
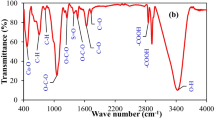
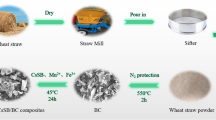
The novel biochar supported by starch and nanoscale iron sulfide (SFeS@Biochar) composites were successfully prepared through coupling of biochar derived from peanut shell with nanoscale ferrous sulfide and starch under nitrogen atmosphere. It had the advantages of biochar, starch, and nanoscale ferrous sulfide. Therefore, it could overcome some shortcomings. The nanoscale ferrous sulfide particles and starch were thought to be loaded successfully on the surface of the biochar by SEM, EDS, BET, XRD, FT-IR, and XPS techniques. High uptake capacity of U(VI) by SFeS@Biochar could be attributed to reactive reaction of FeS nanoparticles and adsorptive of a lot of functional groups. The proposed reaction mechanisms of the U(VI) uptake by SFeS@Biochar were electrostatic attraction, surface complexation, precipitation, and reductive reaction. It also might be an improved environmentally friendly material for U(VI) removal.







Similar content being viewed by others
Availability of data and materials
The data and materials presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
Amuda OS, Giwa AA, Bello IA (2007) Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem Eng J 36:174–181
Budinova T, Ekinci E, Yardim F, Grimm A, Bjornbom E, Minkova V, Goranova M (2006) Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process Technol 87:899–905
Cui P, Liu YH, Cao XH, Li M, Huang GL, Hua R, Wang CX, Liu YT, An XF (2011) Biosorption of uranium(VI) from aqueous solution by dead fungal biomass of penicillium citrinum. Chem Eng J 170:1–6
De Decker J, Folens K, De Clercq J, Meledina M, Van Tendeloo G, Du Laing G, Van Der Voort P (2017) Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption. J Hazard Mater 335:1–9
Donat R, Akdogan A, Erdem E, Centisli H (2005) Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J Colloid Interface Sci 286:43–52
Dong L, Yang J, Mou Y, Sheng G, Wang L, Linghu W, Asiri AM, Alamry KA (2017) Effect of various environmental factors on the adsorption of U(VI) onto biochar derived from rice straw. J Radioanal Nucl Chem 314:377–386
Duan J, Ji HD, Liu W, Zhao X, Han B, Tian ST, Zhao DY (2019) Enhanced immobilization of U(VI) using a new type of FeS-modified Fe0 core-shell particles. Chem Eng J 359:1617–1628
Feng J, Zhu BW, Lim TT (2008) Reduction of chlorinated methanes with nano-scale Fe particles: effects of amphiphiles on the dechlorination reaction and two-parameter regression for kinetic prediction. Chemosphere 73:1817–1823
Feng ML, Sarma D, Qi XH, Du KZ, Huang XY, Kanatzidis MG (2016) Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J. Am. Chem. Soc. 138:12578–12585
Gisuddin AB, Kanel SR, Choi H (2007) Adsorption of humic acid onto nanoscale zero valent iron and its effect on arsenic removal. Environ Sci & Technol 41:2022–2027
Gong Y, Liu Y, **ong Z, Kaback D, Zhao D (2012) Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 23:294007
Guo J, Lua AC (2002) Textural and chemical characterizations of adsorbent prepared from palm shell by potassium hydroxide impregnation at different stages. J Colloid Interface Sci 254:227–233
Hao MJ, Qiu MQ, Yang H, Hu BW, Wang XX (2021) Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Sci Total Environ 760:143333
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221
Hu B, Ye F, Ren X, Zhao D, Sheng G, Li H, Ma J, Wang X, Huang Y (2016) X-ray absorption fine structure study of enhanced sequestration of U(VI) and Se(IV) by montmorillonite decorated with zero-valent iron nanoparticles. Environ Sci Nano 3:1460–1472
Hu BW, Guo XJ, Zheng C, Song G, Chen DY, Zhu YL, Song XF, Sun YB (2019) Plasma-enhanced amidoxime/magnetic graphene oxide for efficient enrichment of U(VI) investigated by EXAFS and modeling techniques. Chem Eng J 357:66–74
Hu BW, Wang HF, Liu RR, Qiu MQ (2021) Highly efficient U(VI) capture by amidoxime/carbon nitride composites: evidence of EXAFS and modeling. Chemosphere 274:129743
Hu Q, Zhu Y, Hu B, Lu S, Sheng G (2018) Mechanistic insights into sequestration of U(VI) toward magnetic biochar: batch, XPS and EXAFS techniques. J Environ Sci 70:217–225
Hua B, Deng B (2008) Reductive immobilization of uranium (VI) by amorphous iron sulfide. Environ Sci Technol 42:8703–8708
Hyun SP, Davis JA, Sun K, Hayes KF (2012) Uranium (VI) reduction by iron (II) monosulfide mackinawite. Environ Sci Technol 46:3369–3376
Jang J, Mirana W, Divine SD, Nawaz M, Shahzad A, Woo SH, Lee DS (2018) Rice straw-based biochar beads for the removal of radioactive strontium from aqueous solution. Sci Total Environ 615:698–707
** J, Li SW, Peng XQ, Liu W, Zhang CL, Yang Y, Han LF, Du ZW, Sun K, Wang XK (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253
Kobayashi N, Okada N, Hirakawa A, Sato T, Hatano JK, Itaya SY, Mori S (2009) Characteristics of solid residues obtained from hot-compressed water treatment of woody biomass. Ind Eng Chem Res 48:373–379
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manage 92:2504–2512
Li MX, Liu HB, Chen TH, Dong C, Sun YB (2019a) Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Sci Total Environ 651:1020–1028
Li ZD, Zhang HQ, **ong XH, Luo F (2019b) U(VI) adsorption onto covalent organic frameworks-TpPa-1. J Solid State Chem 277:484–492
Li ZJ, Chen F, Yuan LY, Liu YL, Zhao YL, Chai ZF, Shi WQ (2012) Uranium(VI) adsorption on graphene oxide nano sheets from aqueous solution. Chem Eng J 210:539–546
Liu H, Li M, Chen T, Chen C, Alharb N, Hayat T, Chen D, Zhang Q, Sun Y (2017) New Synthesis of nZVI/C Composites as an efficient adsorbent for the uptake of U(VI) from aqueous solutions. Environ Sci Technol 51:9227–9234
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. The Innovation 2:100076
Liu J, Valsaraj KT, Devai I, DeLaune R (2008) Immobilization of aqueous Hg (II) by mackinawite (FeS). J Hazard Mater 157:432–440
Lovering JR, Yip A, Nordhaus T (2016) Historical construction costs of global nuclear power reactors. Energy Policy 91:371–382
Lyu HH, Tang JC, Huang Y, Gai LS, Zeng EY, Liber K, Gong YY (2017) Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem Eng J 322:516–524
Ma J, Wang C, ** W, Zhao Q, Wang S, Qiu M, Wang J, Wang X (2021) Removal of radionuclides from aqueous solution by manganese dioxide-based nanomaterials and mechanism research: a review. ACS ES & T Engineering. 1:685–705
Ma S, Huang L, Ma L, Shim Y, Islam SM, Wang P, Zhao LD, Wang S, Sun G, Yang X, Kanatzidis MG (2015) Efficient uranium capture by polysulfide/layered double hydroxide composites. J Am Chem Soc 137:3670–3677
Maity D, Agrawal D (2007) Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater 308:46–55
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J. Colloid Interf. Sci. 296:434–441
Moyes LN, Parkman RH, Charnock JM, Vaughan DJ, Livens FR, Hughes CR, Braithwaite A (2000) Uranium uptake from aqueous solution by interaction with goethite, lepidocrocite, muscovite, and mackinawite: an X-ray absorption spectroscopy study. Environ Sci Technol 34:1062–1068
Newsome J, Morris K, Lloyd JR (2014) The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol 363:164–184
Pang H, Huang S, Wu Y, Yang D, Wang X, Yu S, Chen Z, Alsaedi A, Hayat T, Wang X (2018) Efficient elimination of U(VI) by polyethyleneimine-decorated fly ash. Inorg Chem Front 5:2399–2407
Pang HW, Diao ZF, Wang XX, Ma Y, Yua SJ, Zhu HT, Chen ZS, Hu BW, Chen JR, Wang XK (2019) Adsorptive and reductive removal of U(VI) by Dictyophora indusiate-derived biochar supported sulfide NZVI from wastewater. Chem Eng J 366:368–377
Qiu MQ, Wang M, Zhao QZ, Hu BW, Zhu YL (2018) XANES and EXAFS investigation of uranium incorporation on nZVI in the presence of phosphate. Chemosphere 201:764–771
Qiu MQ, Liu ZX, Wang SQ, Hu BW (2021) The photocatalytic reduction of U(VI) into U(IV) by ZIF-8/g-C3N4 composites at visible light. Environ Res 196:110349
Salvatores M, Palmiotti G (2011) Radioactive waste partitioning and transmutation within advanced fuel cycles: achievements and challenges. Prog Part Nucl Phys 66:144–166
Shi F, Li Y, Zhang Q, Wang H (2012) Synthesis of Fe3O4/C/TiO2 magnetic photocatalyst via vapor phase hydrolysis. Int J Photoenergy 12:1–8
Sun YB, Zhang R, Ding CC, Wang XX, Cheng WC, Chen CL, Wang XK (2016) Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: a combined batch, EXAFS and modeling techniques. Geochim Cosmochim Acta 180:51–65
Troyer LD, Maillot F, Wang Z, Wang Z, Mehta VS, Giammar DE, Catalano JG (2016) Effect of phosphate on U(VI) sorption to montmorillonite: ternary complexation and precipitation barriers. Geochim Cosmochim Acta 175:86–99
Veeramani H, Alessi DS, Suvorova EI, Lezama-Pacheco JS, Stubbs JE, Sharp JO, Dippon U, Kappler A, Bargar JR, Bernier-Latmani R (2011) Products of abiotic U(VI) reduction by biogenic magnetite and vivianite. Geochim Cosmochim Acta 75:2512–2528
Wang H, Guo H, Zhang N, Chen Z, Hu B, Wang X (2019a) Enhanced photoreduction of U(VI) on C3N4 by Cr(VI) and bisphenol A: ESR, XPS, and EXAFS investigation. Environ Sci Technol 53:6454–6461
Wang H, Zhang Q, Qiu M, Hu B (2021) Synthesis and application of perovskite-based photocatalysts in environmental remediation: a review. J Mol Liq 334:116029
Wang XX, Chen L, Wang L, Fan QH, Pan DQ, Li JX, Chi FT, **e Y, Yu SJ, **ao CL, Luo F, Wang J, Wang XL, Chen CL, Wu WS, Shi WQ, Wang SA, Wang XK (2019b) Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci China Chem 62:933–967
Wang X, Dai X, Shi C, Wan J, Silver MA, Zhang L, Chen L, Yi X, Chen B, Zhang D, Yang K, Diwu J, Wang J, Xu Y, Zhou R, Chai Z, Wang S (2019c) A 3,2-Hydroxypyridinone-based decorporation agent that removes uranium from bones in vivo. Nat Commun 10:2570
Wang YQ, Zhang ZB, Liu YH, Cao XH, Liu YT, Li Q (2012) Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem. Eng J 198:246–253
Wolthers M, Van der Gaast SJ, Rickard D (2003) The structure of disordered mackinawite. Am Mineral 88:2007–2015
Yan J, Han L, Gao W, Xue S, Chen M (2014) Biochar supported nanoscale zero valent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol 175:269–274
Yao L, Yang H, Chen ZS, Qiu MQ, Hu BW, Wang XX (2020) Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 273:128576
Yao W, Wu Y, Pang H, Wang X, Yu S, Wang X (2018) In-situ reduction synthesis of manganese dioxide@polypyrrole core/shell nanomaterial for highly efficient enrichment of U(VI) and Eu(III). Sci China Chem 61:812–823
Zelmanov G, Semiat R (2011) Iron (Fe3+) oxide/hydroxide nanoparticles-based agglomerates suspension as adsorbent for chromium (Cr6+) removal from water and recovery. Sep Purif Technol 80:330–337
Zhang H, Liu W, Li A, Zhang D, Li X, Zhai F, Chen L, Chen L, Wang Y, Wang S (2019a) Three mechanisms in one material: uranium capture by a polyoxometalate-organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew Chem Int Ed 58:16110–16114
Zhang J, Chen L, Dai X, Zhu L, **ao C, Xu L, Zhang Z, Alekseev EV, Wang Y, Zhang C, Zhang H, Wang Y, Diwu J, Chai Z, Wang S (2019b) Distinctive two-step intercalation of Sr2+ into a coordination polymer with record High 90Sr uptake capabilities. Chem 5:977–994
Zhang LK, Liu XY, Huang XM, Wang WD, Sun P, Li YM (2019c) Adsorption of Pb2+ from aqueous solutions using Fe-Mn binary oxides-loaded biochar: kinetics, isotherm and thermodynamic studies. Environ Technol 40:1853–1861
Zhang R, Chen C, Li J, Wang X (2015) Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl Surf Sci 349:129–137
Zhang ZB, Cao XH, Liang P, Liu YH (2013) Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonizationm. J Radioanal Nucl Chem 295:1201–1208
Zheng T, Yang Z, Gui D, Liu Z, Wang X, Dai X, Liu S, Zhang L, Gao Y, Chen L, Sheng D, Wang Y, Diwu J, Wang J, Zhou R, Chai Z, Albrecht-Shmitt TE, Wang S (2017) Overcoming the crystallization and designability issues in the ultrastable zirconium phosponate framework system. Nat Commun 8:15369
Zhou Y, Gao B, Zimmerman AR, Chen H, Zhang M, Cao X (2014) Biochar-supported zero valent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 152:538–542
Zong P, Wang S, Zhao Y, Wang H, Pan H, He C (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52
Acknowledgements
The authors are very grateful for the support.
Funding
This work is financially supported by the National Natural Science Foundation of China (No. 21876115) and Natural Science Foundation of Zhejiang Province, China (LGF20C030001 and LGF21C030001).
Author information
Authors and Affiliations
Contributions
Renrong Liu designed the experiment, and Li Han and Hai Wang performed the experiment. Muqing Qiu processed the experimental data and wrote this article. Baowei Hu revised this paper.
Corresponding author
Ethics declarations
Ethics approval
This section is “not applicable” for this study.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 178 kb)
Rights and permissions
About this article
Cite this article
Liu, R., Wang, H., Han, L. et al. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@Biochar composites. Environ Sci Pollut Res 28, 55176–55185 (2021). https://doi.org/10.1007/s11356-021-14835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14835-0