Abstract
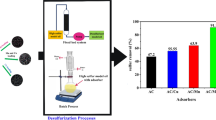
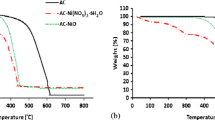
Irradiated waste high-density polyethylene@Zn/ionic liquid novel composite well-fabricated via coacervation method was irradiated by gamma-irradiation and studied the effect of that radiation on the desulfurization process. The prepared composites were characterized by various analytical techniques as follows: X-ray diffraction (XRD), Fourier-Transform infrared (FT-IR), X-ray photoelectron spectrometer (XPS), scanning electron microscope (SEM), High Resolution Transmission Electron Microscopy (HRTEM), N2-adsorption-desorption isotherm, and thermal gravimetric analysis (TG/DTA). The adsorptive desulfurization process of benzothiophene (BT) and dibenzothiophene (DBT) which are harmful compounds in diesel model fuel was investigating using the irradiated and unirradiated composite. The results illustrated that the unirradiated and irradiated composites exhibit an adequate adsorption capacity reached (50–75 mg S/g) and (60–85 mg S/g) for BT and DBT, respectively. The adsorption process over the prepared adsorbents follows the pseudo-second-order kinetic models. The irradiated composite exhibited more adsorption capacity than the unirradiated one due to the radiation generated more surface area and created proton-bond donor sites in the composite surface, which increases the interaction between the surface and sulfur species. The adsorption capacity and adsorption percentage for irradiated and unirradiated composites towards (SCCs) were studied using response surface methodology based on the central composite design (CCD). The thermodynamic factors (∆H°, ∆G°, and ∆S°) reveal that these processes are endothermic adsorption processes. The irradiated PEt @Zn/IL was re-used without significant loss of adsorption activity. This novel irradiated PEt @Zn/IL is the first time used as an adsorbent with an advantage that includes its excellent adsorption capacity, which ensures the product will be efficient in a real process such as the petrochemical industry.














Similar content being viewed by others
Abbreviations
- V :
-
volume of the model oil, (mL)
- W :
-
adsorbent mass, (mg)
- C 0 :
-
the liquid phase sulfur concentration at contact time 0 (initial), (mg S/L)
- C e :
-
the liquid phase sulfur concentration at equilibrium, (mg S/L)
- C t :
-
the liquid phase sulfur concentration at contact time t (min), (mg S/L)
- β 0 :
-
the constant coefficient
- Β :
-
the slope or linear effect of Xi
- X i :
-
input factor
- β ij :
-
the quadratic effect of input factor Xi
- WPEt:
-
waste high-density polyethylene
- ADS:
-
adsorptive desulfurization
- SCCs:
-
sulfur-containing compounds
- CCD:
-
central composite design
- RSM:
-
response surface methodology
- BT:
-
benzothiophene
- DBT:
-
bibenzothiophene
References
Abro R, Abdeltawab AA, Al-Deyab SS, Yu G, Qazi AB, Gao S, Chen X (2014) A review of extractive desulfurization of fuel oils using ionic liquids. RSC Adv 4:35302–35317
Ahmad W, Ahmad I, Ishaq M, Ihsan K (2017) Adsorptive desulfurization of kerosene and diesel oil by Zn impregnated montmorollonite clay. Arab J Chem 10:S3263–S3269
Bagheri M, Masoomi MY, Morsali A (2017) High organic sulfur removal performance of a cobalt based metal-organic framework. J Hazard Mater 331:142–149
Bakry AM, Abbas S, Ali B, Majeed H, Abouelwafa MY, Mousa A, Liang L (2016) Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr Rev Food Sci Food Saf 15:143–182
Balaji Ayyanar C, Marimuthu K (2020) Investigation on the morphology, thermal properties, and in vitro cytotoxicity of the fish scale particulates filled high-density polyethylene composite. Polym Polym Compos 28:285–296
Betiha MA, Rabie AM, Ahmed HS, Abdelrahman AA, El-Shahat MF (2018) Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives: an update review. Egypt J Pet 27:715–730
Chen X, Li Y, Du G, Chen J (2005) Application of response surface methodology in medium optimization for spore production of Coniothyrium minitans in solid-state fermentation. World J Microbiol Biotechnol 21:593–599
Dotto GL, Cadaval T, Pinto L (2012) Use of Spirulina platensis micro and nanoparticles for the removal synthetic dyes from aqueous solutions by biosorption. Process Biochem 47:1335–1343
Elhamifar D, Elhamifar D, Shojaeipoor F (2017) Synthesis, characterization and catalytic application of a novel polyethylene-supported Fe/ionic liquid complex. J Mol Catal A Chem 426:198–204
Elwan HA, Zaky MT, Farag AS, Soliman FS, Hassan MED (2017) A coupled extractive-oxidative process for desulfurization of gasoline and diesel fuels using a bifunctional ionic liquid. J Mol Liq 248:549–555
Fink D (2013) Fundamentals of ion-irradiated polymers, vol 63. Springer-Verlag Berlin Heidelberg, XV, 406. https://doi.org/10.1007/978-3-662-07326-1
Franciski MA, Peres EC, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag 78:630–638
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:e470
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Hameed B, Daud F (2008) Adsorption studies of basic dye on activated carbon derived from agricultural waste: Hevea brasiliensis seed coat. Chem Eng J 139:48–55
Hanrahan G, Lu K (2006) Application of factorial and response surface methodology in modern experimental design and optimization. Crit Rev Anal Chem 36:141–151
Ho Y-S (2003) Removal of copper ions from aqueous solution by tree fern. Water Res 37:2323–2330
Ho Y-S, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Hurisso BB, Lovelock KR, Licence P (2011) Amino acid-based ionic liquids: using XPS to probe the electronic environment via binding energies. Phys Chem Chem Phys 13:17737–17748
Ip AW, Barford JP, McKay G (2010) A comparative study on the kinetics and mechanisms of removal of Reactive Black 5 by adsorption onto activated carbons and bone char. Chem Eng J 157:434–442
Jiang B, Tantai X, Zhang L, Hao L, Sun Y, Deng L, Shi Z (2015) Synthesis of chlorostannate (II) ionic liquids and their novel application in the preparation of high-quality L-lactide. RSC Adv 5:50747–50755
Joglekar A, May A (1987) Product excellence through design of experiments. Cereal Foods World 32:857-&
Jung MR, Horgen FD, Orski SV, Rodriguez V, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer S-J (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull 127:704–716
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2009) Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon. Desalination 238:210–232
Kim S, Park C, Lee J (2020) Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J Hazard Mater 392:122464
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lee KX, Valla JA (2019) Adsorptive desulfurization of liquid hydrocarbons using zeolite-based sorbents: a comprehensive review. React Chem Eng 4:1357–1386
Li Y-X, Jiang W-J, Tan P, Liu X-Q, Zhang D-Y, Sun L-B (2015) What matters to the adsorptive desulfurization performance of metal-organic frameworks? J Phys Chem C 119:21969–21977
Mamunya YP, Zois H, Apekis L, Lebedev E (2004) Influence of pressure on the electrical conductivity of metal powders used as fillers in polymer composites. Powder Technol 140:49–55
Matloob AM, Abd El-Hafiz DR, Saad L, Mikhail S, Guirguis D (2019) Metal organic framework-graphene nano-composites for high adsorption removal of DBT as hazard material in liquid fuel. J Hazard Mater 373:447–458
Mohebali G, Ball AS (2016) Biodesulfurization of diesel fuels–past, present and future perspectives. Int Biodeterior Biodegradation 110:163–180
Mujahid M, Singh P, Srivastava D, Gupta S, Avasthi D, Kanjilal D (2004) Study of chain scission versus crosslinking in MeV ion-irradiated polycarbonate using dielectric constant measurements and UV spectroscopy. Radiat Meas 38:197–203
Myers RH, Montgomery DC, Anderson-Cook CM (1995) Response surface methodology: process and product optimization using designed experiments. Wiley, New York, pp 134–174
Na R, Huo G, Zhang S, Huo P, Du Y, Luan J, Zhu K, Wang G (2016) A novel poly (ethylene glycol)–grafted poly (arylene ether ketone) blend micro-porous polymer electrolyte for solid-state electric double layer capacitors formed by incorporating a chitosan-based LiClO 4 gel electrolyte. J Mater Chem A 4:18116–18127
Nazal MK, Oweimreen GA, Khaled M, Atieh MA, Aljundi IH, Abulkibash AM (2016) Adsorption isotherms and kinetics for dibenzothiophene on activated carbon and carbon nanotube doped with nickel oxide nanoparticles. Bull Mater Sci 39:437–450
Nefedieva M, Lebedeva O, Kultin D, Kustov L, Borisenkova S, Krasovskiy V (2010) Ionic liquids based on imidazolium tetrafluoroborate for the removal of aromatic sulfur-containing compounds from hydrocarbon mixtures. Green Chem 12:346–349
Wendy C, Phase I, Emissions PM (1999) Diesel Emission Control–Sulfur Effects (DECSE) Program
Prasad AL, Santhi T, Manonmani S (2015) Recent developments in preparation of activated carbons by microwave: Study of residual errors. Arab J Chem 8:343–354
Preetha R, Jayaprakash N, Philip R, Bright Singh I (2007) Optimization of carbon and nitrogen sources and growth factors for the production of an aquaculture probiotic (Pseudomonas MCCB 103) using response surface methodology. J Appl Microbiol 102:1043–1051
Raghu S, Archana K, Sharanappa C, Ganesh S, Devendrappa H (2016) Electron beam and gamma ray irradiated polymer electrolyte films: Dielectric properties. J Radiat Res Appl Sci 9:117–124
Saha B, Vedachalam S, Dalai AK (2020) Review on recent advances in adsorptive desulfurization. Fuel Process Technol 214:106685
Saleh TA, Sulaiman KO, AL-Hammadi SA, Dafalla H, Danmaliki GI (2017) Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: with column system evaluation. J Clean Prod 154:401–412
Sarker M, Rashid MM, Molla M, Rahman M (2011) High density polyethylene (HDPE-2) and polyethylene (PS-6) waste Plastic mixture turn into valuable fuel energy. J Int Sci Publ Mater Methods Tech 5:1313
Shi Y, Zhang X, Liu G (2015) Activated carbons derived from hydrothermally carbonized sucrose: remarkable adsorbents for adsorptive desulfurization. ACS Sustain Chem Eng 3:2237–2246
Silva J, Farias B, Gründmann D, Cadaval T Jr, Moura J, Dotto G, Pinto L (2017) Development of chitosan/Spirulina bio-blend films and its biosorption potential for dyes. J Appl Polym Sci 134:44580
Song C, Ma X (2003) New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl Catal B Environ 41:207–238
Susastriawan A, Sandria A (2020) Experimental study the influence of zeolite size on low-temperature pyrolysis of low-density polyethylene plastic waste. Therm Sci Eng Prog 17:100497
Swat AAA, Saleh TA, Ganiyu SA, Siddiqui MN, Alhooshani KR (2017) Preparation of activated carbon, zinc oxide and nickel oxide composites for potential application in the desulfurization of model diesel fuels. J Anal Appl Pyrolysis 128:246–256
Thaligari SK, Srivastava VC, Prasad B (2016) Adsorptive desulfurization by zinc-impregnated activated carbon: characterization, kinetics, isotherms, and thermodynamic modeling. Clean Techn Environ Policy 18:1021–1030
Villar-Garcia IJ, Smith EF, Taylor AW, Qiu F, Lovelock KR, Jones RG, Licence P (2011) Charging of ionic liquid surfaces under X-ray irradiation: the measurement of absolute binding energies by XPS. Phys Chem Chem Phys 13:2797–2808
Wang F, Zhang Z, Yang J, Wang L, Lin Y, Wei Y (2013) Immobilization of room temperature ionic liquid (RTIL) on silica gel for adsorption removal of thiophenic sulfur compounds from fuel. Fuel 107:394–399
Wang J, Zhang L, Sun Y, Jiang B, Chen Y, Gao X, Yang H (2018) Deep catalytic oxidative desulfurization of fuels by novel Lewis acidic ionic liquids. Fuel Process Technol 177:81–88
Whitcomb PJ, Anderson MJ (2004) RSM simplified: optimizing processes using response surface methods for design of experiments. Taylor & Francis Incorporated, CRC press
Whitcomb PJ, Anderson MJ (2016) Rsm simplified-optimizing processes using response surface methods for de. Taylor & Francis Incorporated, CRC press
Wu Z, Ondruschka B (2010) Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason Sonochem 17:1027–1032
Wu B, Deng L, Gu W, Wu B, Guo J (2019) Experimental investigation of combustion and particle emissions under different combustion modes on a heavy-duty diesel engine fueled by diesel/gasoline/diesel from direct coal liquefaction. Fuel 254:115661
Yang K, Yan Y, Chen W, Kang H, Han Y, Zhang W, Fan Y, Li Z (2018) The high performance and mechanism of metal–organic frameworks and their composites in adsorptive desulfurization. Polyhedron 152:202–215
Yaseen M, Ullah S, Ahmad W, Subhan S, Subhan F (2021) Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel 284:119102
Yildiz I, Açıkkalp E, Caliskan H, Mori K (2019) Environmental pollution cost analyses of biodiesel and diesel fuels for a diesel engine. J Environ Manag 243:218–226
You N, Wang X-F, Li J-Y, Fan H-T, Shen H, Zhang Q (2019) Synergistic removal of arsanilic acid using adsorption and magnetic separation technique based on Fe3O4@ graphene nanocomposite. J Ind Eng Chem 70:346–354
Yurdakal S, Garlisi C, Özcan L, Bellardita M (2019) Heterogeneous photocatalysis relationships with heterogeneous catalysis and perspectives, (Photo)catalyst characterization techniques. Adsorption Isotherms and BET, SEM, FTIR, UV–Vis, Photoluminescence, and Electrochemical Characterizations, pp 87–152. https://doi.org/10.1016/B978-0-444-64015-4.00004-3
Zhang J, Sun S, Bian Y, Li W, Liu R, Zhao D (2018a) Adsorptive desulfurization of metal phthalocyanine functionalized poly-ionic liquids grafted to silica gel. Fuel 220:513–520
Zhang X-F, Wang Z, Feng Y, Zhong Y, Liao J, Wang Y, Yao J (2018b) Adsorptive desulfurization from the model fuels by functionalized UiO-66 (Zr). Fuel 234:256–262
Zhao S, He M, Zhou Y, Sheng X, Fu X, Zhang Y (2015) Synthesis of micro/mesoporous silica material by dual-template method as a heterogeneous catalyst support for alkylation. RSC Adv 5:28124–28132
Zhao S, Zhang Y, Zhou Y, Zhang C, Sheng X, Fang J, Zhang M, Yang Y (2017) Ionic liquid-assisted synthesis of highly dispersive bowknot-like ZnO microrods for photocatalytic applications. Appl Surf Sci 400:269–276
Zhu W, Wu P, Yang L, Chang Y, Chao Y, Li H, Jiang Y, Jiang W, Xun S (2013) Pyridinium-based temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels. Chem Eng J 229:250–256
Zois H, Apekis L, Mamunya YP (2003) Structure-electrical properties relationships of polymer composites filled with Fe-powder, Macromolecular Symposia. Wiley Online Library, pp. 351-359
Zou X, Zhou W, Shi J, Ye Y, Zhao Y, Zhang H, Liu Y, Yu Y, Guo J (2020) Preparation and characterization of poly (N-methylol acrylamide)/polyethylene glycol composite phase change materials for thermal energy storage. Sol Energy Mater Sol Cells 205:110248
Author information
Authors and Affiliations
Contributions
E.G. Zaki: methodology, conceptualization, writing—review and editing, and original draft. Dina Mohmed: conceptualization, methodology, software, data curation, and writing. Delvin Aman: methodology, conceptualization visualization, and writing—review and editing original draft. Modather F. Hussein: software, data curation, and writing. Fathi S. Soliman: Software, Visualization. M. M. El-Zayat: methodology, conceptualization, and writing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 329 kb).
Rights and permissions
About this article
Cite this article
Zaki, E.G., Mohmed, D., Hussein, M.F. et al. Assessment of polyethylene/Zn-ionic as a diesel fuel sulfur adsorbent: gamma radiation effect and response surface methodology. Environ Sci Pollut Res 28, 52993–53009 (2021). https://doi.org/10.1007/s11356-021-14501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14501-5