Abstract
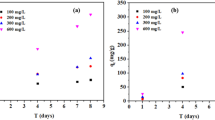
High industrialization and improved medical facilities are deteriorating aquatic bodies through untreated effluents. This study is aimed to design and characterize the bentonite, Duranta erecta, and their hybrid-alginate beads for the removal of cetyltrimethylammonium bromide (CTAB) from its aqueous solution. D. erecta’s seed powder was treated by using a sonochemical method and embedded into alginate beads. All designed beads were characterized by using physicochemical methods, Fourier-transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD) technique. Hybrid beads were found to form an appropriate hydrogel structure with maximum surface area per unit gram (544 cm2 g−1), 0.42 mg dry weight, and 2.70 mm diameter. Kinetics and intraparticle diffusion models were fitted where involvement of both chemisorption and intraparticle diffusion was observed during the initial 30 and post-30-min phase, respectively. Thermodynamic studies corroborated the spontaneity of the CTAB adsorption process. Bentonite alginate beads showed the highest adsorption capacity of 97.06 mg g−1 in 100 mg L−1 CTAB solution at optimized conditions, while hybrid-alginate beads showed excellent efficiency with a wide range of physicochemical conditions frame. Conclusively, designed beads can be used to remove the surfactant, i.e., CTAB, from industrial waste effluents for the betterment of water reservoirs.









Similar content being viewed by others
References
Abd El-Gawad HS (2014) Aquatic environmental monitoring and removal efficiency of detergents. Water Sci 28:51–64. https://doi.org/10.1016/j.wsj.2014.09.001
Abd El-Lateef HM, Ali MMK, Saleh MM (2018) Adsorption and removal of cationic and anionic surfactants using zero-valent iron nanoparticles. J Mol Liq 268:497–505. https://doi.org/10.1016/j.molliq.2018.07.093
Aboul-Maaty NA-F, Oraby HA-S (2019) Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull Natl Res Cent 43:25. https://doi.org/10.1186/s42269-019-0066-1
Alabarse FG, Conceição RV, Balzaretti NM, Schenato F, Xavier AM (2011) In-situ FTIR analyses of bentonite under high-pressure. Appl Clay Sci 51:202–208. https://doi.org/10.1016/j.clay.2010.11.017
Ali R (2020) Removal of heavy metals from aqueous media by biosorption. Arab J Basic Appl Sci 27:183–193. https://doi.org/10.1080/25765299.2020.1756177
Alkan M, Karadaş M, Doğan M, Demirbaş Ö (2005) Adsorption of CTAB onto perlite samples from aqueous solutions. J Colloid Interface Sci 291:309–318. https://doi.org/10.1016/j.jcis.2005.05.027
Alves DC, Goncalves JO, Coseglio BB, Burgo TA, Dotto GL, Pinto LA, Cadaval TR Jr (2019) Adsorption of phenol onto chitosan hydrogel scaffold modified with carbon nanotubes. J Environ Chem Eng 7:103460. https://doi.org/10.1016/j.jece.2019.103460
Bae S, Mannan MB, Lee W (2012) Adsorption of cationic cetylpyridinium chloride on pyrite surface. J Ind Eng Chem 18:1482–1488. https://doi.org/10.1016/j.jiec.2012.02.010
Bautista-Toledo MI, Rivera-Utrilla, J, Méndez-Díaz JD, Sánchez-Polo M, Carrasco-Marín F (2014) Removal of the surfactant sodium dodecylbenzenesulfonate from water by processes based on adsorption/bioadsorption and biodegradation. J Colloid Interface Sci 418:113–119. https://doi.org/10.1016/j.jcis.2013.12.001
Chang L, Cao Y, Peng W, Li C, Fan G, Song X, Shi X (2020) Efficiently removing cetyl trimethyl ammonium bromide from wastewater by graphene oxide. Surf Interface Anal 52:611–619. https://doi.org/10.1002/sia.6800
Chelli VR, Golder AK (2018) Ag-do** on ZnO support mediated by bio-analytes rich in ascorbic acid for photocatalytic degradation of dipyrone drug. Chemosphere 208:149–158. https://doi.org/10.1016/j.chemosphere.2018.05.158
Chelli VR, Chakraborty S, Golder AK (2018) Ag-do** on TiO2 using plant-based glycosidic compounds for high photonic efficiency degradative oxidation under visible light. J Mol Liq 271:380–388. https://doi.org/10.1016/j.molliq.2018.08.140
Darvishi Z, Morsali A (2011) Synthesis and characterization of nano-bentonite by sonochemical method. Ultrason Sonochem 18:238–242. https://doi.org/10.1016/j.ultsonch.2010.05.012
El Bouraie M, Masoud AA (2017) Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg (OH) 2. Appl Clay Sci 140:157–164. https://doi.org/10.1016/j.clay.2017.01.021
Eskandari-Nojehdehi M, Jafarizadeh-Malmiri H, Rahbar-Shahrouzi J (2016) Optimization of processing parameters in green synthesis of gold nanoparticles using microwave and edible mushroom (Agaricus bisporus) extract and evaluation of their antibacterial activity. Nanotechnol Rev 5:537–548. https://doi.org/10.1515/ntrev-2016-0064
Fotsing PN, Woumfo ED, Măicăneanu SA, Vieillard J, Tcheka C, Ngueagni PT, Siéwé JM (2020) Removal of Cu (II) from aqueous solution using a composite made from cocoa cortex and sodium alginate. Environ Sci Pollut Res 27:8451–8466. https://doi.org/10.1007/s11356-019-07206-3
Giri AS, Golder AK (2019) Ciprofloxacin degradation in photo-Fenton and photo-catalytic processes: degradation mechanisms and iron chelation. Int J Environ Sci 80:82–92. https://doi.org/10.1016/j.jes.2018.09.016
Han S-F, ** W, Tu R, Ding B, Zhou X, Gao S-h, Feng X, Yang Q, Wang Q (2020) Screening and mutagenesis of high-efficient degrading bacteria of linear alkylbenzene sulfonates. Chemosphere 245:125559. https://doi.org/10.1016/j.chemosphere.2019.125559
Hao L, Wang N, Wang C, Li G (2018) Arsenic removal from water and river water by the combined adsorption-UF membrane process. Chemosphere 202:768–776. https://doi.org/10.1016/j.chemosphere.2018.03.159
He J, Unser S, Bruzas I, Cary R, Shi Z, Mehra R, Aron K, Sagle L (2018) The facile removal of CTAB from the surface of gold nanorods. Colloids Surf B: Biointerfaces 163:140–145. https://doi.org/10.1016/j.colsurfb.2017.12.019
Hussein LAA, Fares NV, El-Kosasy AM (2014) Spectrophotometric, spectrofluorimetric, and potentiometric assays of cetyltrimethylammonium bromide in industrial wastewater samples. J AOAC Int 97:1175–1182. https://doi.org/10.5740/jaoacint.13-102
Jawed A, Saxena V, Pandey LM (2020) Engineered nanomaterials and their surface functionalization for the removal of heavy metals: a review. J Water Process Eng 33:101009. https://doi.org/10.1016/j.jwpe.2019.101009
Koner S, Pal A, Adak A (2010) Cationic surfactant adsorption on silica gel and its application for wastewater treatment. Desalination Water Treat 22:1–8. https://doi.org/10.5004/dwt.2010.1465
Kruszelnicka I, Ginter-Kramarczyk D, Wyrwas B, Idkowiak J (2019) Evaluation of surfactant removal efficiency in selected domestic wastewater treatment plants in Poland. J Environ Health Sci Eng 1-8. https://doi.org/10.1007/s40201-019-00387-6
Li Z (2007) Removal of cationic surfactants from water using clinoptilolite zeolite. Stud Surf Sci Catal 170:2098–2103. https://doi.org/10.1016/S0167-2991(07)81105-0
Mojoudi N, Mirghaffari N, Soleimani M, Shariatmadari H, Belver C, Bedia J (2019) phenol adsorption on high microporous activated carbons prepared from oily sludge: equilibrium, kinetic and thermodynamic studies. Scientif Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-55794-4
Obayomi K, Bello J, Yahya M, Chukwunedum E, Adeoye J (2020) Statistical analyses on effective removal of cadmium and hexavalent chromium ions by multiwall carbon nanotubes (MWCNTs). Heliyon 6:e04174. https://doi.org/10.1016/j.heliyon.2020.e04174
Prasad M, Saxena S (2004) Sorption mechanism of some divalent metal ions onto low-cost mineral adsorbent. Ind Eng Chem Res 43:1512–1522. https://doi.org/10.1021/ie030152d
Puri AV (2018) Duranta Repens Linn.(Verbenaceae): a comprehensive review of pharmacognostic, Ethnomedicinal, Pharmacological, And Phytochemical Aspects. Asian J Pharm Clin Res 11(11):91–96. https://doi.org/10.22159/ajpcr.2018.v11i11.28509
Ravi, Pandey LM (2019) Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl Clay Sci 169:102–111. https://doi.org/10.1016/j.clay.2018.12.019
Saouter E, Pittinger C, Feijtel T (2001) Aquatic environmental impact of detergents: from simple to more sophisticated models. Ecotoxicol Environ Saf 50:153–159. https://doi.org/10.1006/eesa.2001.2084
Shami S, Dash RR, Verma AK, Dash AK, Pradhan A (2020) Adsorptive removal of surfactant using dolochar: a kinetic and statistical modeling approach. Water Environ Res 92:222–235. https://doi.org/10.1002/wer.1193
Sharma S, Hasan A, Kumar N, Pandey LM (2018) Removal of methylene blue dye from aqueous solution using immobilized Agrobacterium fabrum biomass along with iron oxide nanoparticles as biosorbent. Environ Sci Pollut Res 1-11:21605–21615. https://doi.org/10.1007/s11356-018-2280-z
Siyal AA, Shamsuddin R, Low A, Hidayat A (2020) Adsorption kinetics, isotherms, and thermodynamics of removal of anionic surfactant from aqueous solution using fly ash. Water Air Soil Pollut 231:1–13. https://doi.org/10.1007/s11270-020-04879-2
Subsongsang R, Jiraungkoorskul W (2016) An updated review on phytochemical properties of “Golden Dewdrop” Duranta erecta. Pharmacogn Rev 10(20):115. https://doi.org/10.4103/0973-7847.194042
Takayanagi A, Kobayashi M, Kawase Y (2017) Removal of anionic surfactant sodium dodecyl benzene sulfonate (SDBS) from wastewaters by zero-valent iron (ZVI): predominant removal mechanism for effective SDBS removal. Environ Sci Pollut Res 24:8087–8097. https://doi.org/10.1007/s11356-017-8493-8
Touny AH, Abd El-Lateef HM, Saleh MM (2019) Removal of cationic surfactants from dilute solutions using nanoporous nickel phosphate: a structural, kinetic and thermodynamic study. J Mol Liq 283:30–38. https://doi.org/10.1016/j.molliq.2019.03.032
Vilela PB, Dalalibera A, Duminelli EC, Becegato VA, Paulino AT (2019) Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ Sci Pollut Res 26:28481–28489. https://doi.org/10.1007/s11356-018-3208-3
Yahya MD, Obayomi KS, Abdulkadir MB, Iyaka YA, Olugbenga AG (2020) Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium. Water Sci Eng 13:202–213. https://doi.org/10.1016/j.wse.2020.09.007
Yalçin M, Gürses A, Doğar Ç, Sözbili M (2005) The adsorption kinetics of cethyltrimethylammonium bromide (CTAB) onto powdered active carbon. Adsorption 10:339–348. https://doi.org/10.1007/s10450-005-4819-9
Acknowledgements
The authors would like to acknowledge the Department of Chemical Engineering and Central Instruments Facility, Indian Institute of Technology Guwahati, for providing all logistic and analytical facilities for this work. Soma Chauhan would like to thank the University Institute of Engineering and Technology, Kurukshetra University, Kurukshetra, Haryana, for granting permission to carry out this work at the Indian Institute of Technology Guwahati.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
Conceptualization: Dr. Animes Kumar Golder, Soma Chauhan, and Ravi Ravi; methodology: Soma Chauhan and Ravi Ravi; formal analysis and investigation: Soma Chauhan (major contribution) and Ravi Ravi; writing—original draft preparation: Ravi Ravi (major contribution) and Soma Chauhan; writing—review and editing: Dr. Animes Kumar Golder; funding acquisition: Dr. Animes Kumar Golder; resources: Dr. Animes Kumar Golder, Soma Chauhan, and Ravi Ravi; supervision: Dr. Animes Kumar Golder.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bentonite/Duranta erecta’s fruit powder imbedded alginate beads effective removal of cetyltrimethylammonium bromide.
• Bentonite-alginate beads showed maximum adsorption capacity of 2862 mg g−1.
• Removal involves rapid surface adsorption followed by intraparticle diffusion.
• Kinetic studies and thermodynamic studies confirmed chemisorption and spontaneous nature of adsorption.
Rights and permissions
About this article
Cite this article
Golder, A.K., Chauhan, S. & Ravi, R. Synthesis of low-cost bentonite/Duranta erecta’s fruit powder imbedded alginate beads and its application in surfactant removal. Environ Sci Pollut Res 28, 58945–58957 (2021). https://doi.org/10.1007/s11356-021-14306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14306-6