Abstract
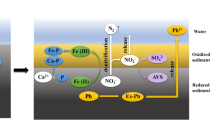
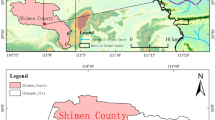
To estimate the pollution of As and Pb in the Songhua River which flows through the major rice-producing regions in China, the present study investigated the level and release of As and Pb in surficial sediments which collected from nine sites in Songhua River (M1–M9). The concentration of As and Pb was ranged as follows: As = 3.104~15.01 μg/g, Pb = 20.10~37.42 μg/g; the average concentration: As = 6.466 ± 3.077 μg/g, Pb = 28.88 ± 5.077 μg/g. By analysis vertically, the average concentration of As was 5.166 ± 1.496 μg/g in the upstream, 5.815 ± 1.793 μg/g in the midstream, and 9.716 ± 4.977 μg/g in the downstream. The average concentration of Pb was 27.83 ± 4.552 μg/g in the upstream, 28.66 ± 6.333 μg/g in the midstream, and 30.99 ± 4.837 μg/g in the downstream. It indicated that the concentration of As and Pb increased gradually from upstream to downstream. As existed mainly as insoluble state and Pb existed mainly as sulfide and organic combining state in surficial sediments, and the species of As and Pb could transform with the change of the circumstance. The release of quantity of As was higher than Pb. The pH of 6 was not conducive to the release of As and Pb. When the temperature was 35 and 6 °C, the release of As and Pb in surficial sediments were restrained, respectively. Fumaric acid and citric acid played an important role in promoting the release of As, but not conducive to Pb. Furthermore, the reasonable aeration rate was beneficial to the release process of As and Pb in surficial sediment. By kinetic analysis, the Elovich equation (Ct = 84.931–8.952lnt) could be used to describe the dynamic process of the release of As in a relatively short time. The Elovich equation (C t = 2.724 + 1.3724lnt) and double constant rate equation (lnC T = 1.4646 + 0.1522lnT) could well describe the dynamics process of the release of Pb.










Similar content being viewed by others
References
Arribére MA et al (2003) Heavy metals in the vicinity of a chlor-alkali factory in the upper Negro River ecosystem, Northern Patagonia, Argentina. Sci Total Environ 301(1–3):187–203
Barnhart J (1997) Occurrences, uses, and properties of chromium. Regul Toxicol Pharmacol 26(2):3–7
Beneš P, Čejchanová M, Havlík B (1985) Migration and speciation of lead in a river system heavily polluted from a smelter. Water Res 19(1):1–6
Braman RS, Foreback CC (1973) Methylated forms of arsenic in the environment. Science 182(4118):1247–1249
Calmano W, Hong J, Förstner U (1993) Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol 28(8–9):223–235
Chartier M, Mercier G, Blais JF (2001) Partitioning of trace metals before and after biological removal of metals from sediments. Water Res 35(6):1435–1444
Dudka S, Adriano DC (1997) Environmental impacts of metal ore mining and processing: a review. J Environ Qual 26(3):516–528
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30(7):973–980
Farag AM et al (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d'Alene River Basin, Idaho. Arch Environ Contam Toxicol 34(2):119–127
Guo SH et al (2006) Investigation on Fe, Mn, Zn, Cu, Pb and Cd fractions in the natural surface coating samples and surficial sediments in the Songhua River, China. J Environ Sci 18(6):1193–1198
Hahn C, Trang Hoang TQ (2015) Arsenic fractionation in agricultural soil in Vietnam using the Sequential extraction procedure. Int Conf Inform Environ Energy Appl 2015:2184–2199
Han, Futao, **anhuai Huang, and Jianshe Tang 2014 Study on pollution and release characteristics of heavy metals from the river sediments. Ind Saf Environ Prot
Hui Z et al (2011) Geochemical characteristics of heavy metals in riparian sediment pore water of Songhua River, Northeast China. Chin Geogr Sci 61(2):195–203
Jain CK, Sharma MK (2001) Distribution of trace metals in the Hindon River system, India. J Hydrol 253(1–4):81–90
Kiikkilä O et al (2001) In situ bioremediation through mulching of soil polluted by a copper-nickel smelter. J Environ Qual 30(4):1134–1143
Lin C et al (2008) Distribution and contamination assessment of heavy metals in sediment of the second Songhua River, China. Environ Monit Assess 137(1–3):329–342
Lion LW, Altmann RS, Leckie JO (1982) Trace-metal adsorption characteristics of estuarine particulate matter: evaluation of contributions of Fe/Mn oxide and organic coatings. Environ Sci Technol 16(10)
Mclintock IS (1967) The Elovich equation in chemisorption kinetics. Nature 216(5121):1204–1205
Onat B, Şahin ÜA (2014) An assessment of particulate mercury and arsenic concentrations in size-fractioned total suspended particulate matter in urban areas. Air Qual Atmos Health 7(2):131–141
Peng JF et al (2008) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2–3):633–640
Peng Y et al (2014) Contamination assessment and release kinetics of heavy metals in sediments of urban-rural ecotones. Fresenius Environ Bull 23(8):1785–1789
Rausa R, Mascolo G, Bassetti A (1999) Thermal treatment of sediments as a function of temperature and reacting atmosphere. J Anal Appl Pyrolysis 49(1–2):425–445
Salomons W, Stigliani WM (1995) Biogeodynamics of pollutants in soils and sediments. Springer-Verlag, Dordrecht
Santschi PH, Lenhart JJ, Honeyman BD (1997) Heterogeneous processes affecting trace contaminant distribution in estuaries: the role of natural organic matter. Mar Chem 58(1):99–125
Tessier A, Campbell P, and Bisson M 1979 Sequential extraction procedures speciation of particulate trace metals
Vuceta J, Morgan JJ (1978) Chemical modeling of trace metals in fresh waters: role of complexation and adsorption. Environ Sci Technol 13(2):239–239
Wang C et al (2010a) Speciation analysis of metals (Tl, Cd and Pb) in Tl-containing pyrite and its cinder from Yunfu Mine, China, by ICP-MS with sequential extraction. Chin J Geochem 29(1):113–119
Wang L et al (2013) Biosorption of Pb(II) and Zn(II) by extracellular polymeric substance (Eps) of Rhizobium radiobacter: equilibrium, kinetics and reuse studies. Arch Environ Prot 39(2):129–140
Wang N et al (2010b) Distribution of methyl mercury in Rana chensinensis and environmental media in gold-mining areas of upper reaches of Songhua River, China. Chin Geogr Sci 20(4):330–336
Yang S et al (2011) Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ Sci Technol 45(8):3621–3627
Yu KC et al (1996) Remobilization of zinc from Ell-Ren River sediment fractions affected by EDTA, DTPA and EGTA. Water Sci Technol 34(7):125–132
Zhang KL et al (2009a) Syntheses, characterization and luminescent properties of two lead(II) fumarate metal-organic frameworks. Polyhedron 28(4):647–652
Zhang KL et al (2009b) Synthesis and characterization of one lead(II) photoluminescent coordination polymer with the fumarate ligand. Inorg Chim Acta 362(11):4255–4259
Zoumis T et al (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266(1–3):195–202
Acknowledgments
This work was supported by China’s ministry of environmental protection commonweal special industry research (Grant No. 2010467038), Heilongjiang provincial environmental science research institution, the Key Laboratory of Superlight Materials and Surface Technology of Ministry of Education and Harbin, Engineering University. This study was also supported by the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. QAK201715).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Liu, GM., An, Q., Wang, LJ. et al. Release and kinetics of arsenic and plumbum in the Songhua River surficial sediments. Environ Sci Pollut Res 25, 541–551 (2018). https://doi.org/10.1007/s11356-017-0365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0365-8