Abstract
Purpose
Oral squamous cell carcinoma (OSCC) has not seen a substantial improvement in patient survival despite therapeutic advances, making accurate detection and characterization of the disease a clinical priority. Here, we aim to demonstrate the effectiveness of magnetic resonance imaging (MRI) with the targeted MRI contrast agent MT218 specific to extradomain-B fibronectin (EDB-FN) in the tumor microenvironment for detection and characterization of aggressive OSCC tumors.
Procedures
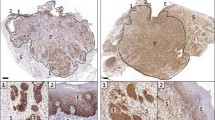
EDB-FN expression was evaluated in human normal tongue and OSCC specimens with immunohistochemistry. Invasiveness of human CAL27, HSC3, and SCC4 OSCC cells was analyzed with spheroid formation and transwell assays. EDB-FN expression in the cells was analyzed with semiquantitative real-time PCR, western blotting, and a peptide binding study with confocal microscopy. Contrast-enhanced MRI with MT218 was performed on subcutaneous OSCC mouse models at a dose of 0.04 mmol/kg, using gadoteridol (0.1 mmol/kg) as a control.
Results
Strong EDB-FN expression was observed in human untreated primary and metastatic OSCC, reduced expression in treated OSCC, and little expression in normal tongue tissue. SCC4 and HSC3 cell lines demonstrated high invasive potential with high and moderate-EDB-FN expression, respectively, while CAL27 showed little invasive potential and low-EDB-FN expression. In T1-weighted MRI, MT218 produced differential contrast enhancement in the subcutaneous tumor models in correlation with EDB-FN expression in the cancer cells. Enhancement in the high-EDB-FN tumors was greater with MT218 at 0.04 mmol/kg than gadoteridol at 0.1 mmol/kg.
Conclusions
The results suggest EDB-FN has strong potential as an imageable biomarker for aggressive OSCC. MRMI results demonstrate the effectiveness of MT218 and the potential for differential diagnostic imaging of oral cancer for improving the management of the disease.






Similar content being viewed by others
References
Rivera C (2015) Essentials of oral cancer. Int J Clin Exp Pathol 8:11884–11894
Chen SW, Zhang Q, Guo ZM, Chen WK, Liu WW, Chen YF, Li QL, Liu XK, Li H, Ou-Yang D, Chen WC, Fu XY, Wang XD, Yang AK, Bei JX, Song M (2018) Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag Res 10:4523–4535
Sarode GS, Sarode SC, Maniyar N et al (2019) Recent trends in predictive biomarkers for determining malignant potential of oral potentially malignant disorders. Oncol Rev 13:424
Seoane-Romero JM, Vázquez-Mahía I, Seoane J et al (2012) Factors related to late stage diagnosis of oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 17:35–40
Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY (2005) Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 29:167–178
Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Gilbert J, Haddad RI, Hicks WL Jr, Hitchcock YJ, Jimeno A, Leizman D, Lydiatt WM, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco J, Rodriguez CP, Shah JP, Weber RS, Witek M, Worden F, Yom SS, Zhen W, Burns JL, Darlow SD (2017) NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Cancer Netw 15:761–770
Cho JH, Lee YS, Sun DI, Kim MS, Cho KJ, Nam IC, Kim CS, Kim SY, Park YH, Joo YH (2016) Prognostic impact of lymph node micrometastasis in oral and oropharyngeal squamous cell carcinoma. Head Neck 38(Suppl 1):E1777–E1782
Lingen MW, Tampi MP, Urguhart O et al (2017) Adjuncts for the evaluation of potentially malignant disorders in the oral cavity: diagnostic test accuracy systematic review and meta-analysis-a report of the American Dental Association. J Am Dent Assoc 148:797–813
Pałasz P, Adamski Ł, Górska-Chrzastek M, Starzyńska A, Studniarek M (2017) Contemporary diagnostic imaging of oral squamous cell carcinoma – a review of literature. Pol J Radiol 82:193–202
Seeburg DP, Baer AH, Aygun N (2018) Imaging of patients with head and neck cancer: from staging to surveillance. Oral Maxillofac Surg Clin N Am 30:421–433
Abraham J (2015) Imaging for head and neck cancer. Surg Oncol Clin N Am 24:455–471
Zhou Z, Lu ZR (2013) Gadolinium-based contrast agents for MR cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:1–18
Zhou Z, Lu ZR (2017) Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev 113:24–48
Han Z, Lu ZR (2017) Targeting fibronectin for cancer imaging and therapy. J Mater Chem B 5:639–654
Ayat NR, Qin JC, Cheng H, Roelle S, Gao S, Li Y, Lu ZR (2018) Optimization of ZD2 peptide targeted Gd (HP-DO3A) for detection and risk-stratification of prostate cancer with MRI. ACS Med Chem Lett 9:730–735
Ayat NR, Vaidya A, Yeung GA, Buford MN, Hall RC, Qiao PL, Yu X, Lu ZR (2019) Effective MR molecular imaging of triple negative breast cancer with an EDB-fibronectin-specific contrast agent at reduced doses. Front Oncol 9:1351
Birchler MT, Milisavlijevic D, Pfaltz M, Neri D, Odermatt B, Schmid S, Stoeckli SJ (2003) Expression of the extra domain B of fibronectin, a marker of angiogenesis, in head and neck tumors. Laryngoscope 113:1231–1237
Vaidya AM, Wang H, Qian V, Gilmore H, Lu ZR (2020) Overexpression of extradomain-B fibronectin is associated with invasion of breast cancer cells. Cells 9:1826
Han Z, Zhou Z, Shi X, Wang J, Wu X, Sun D, Chen Y, Zhu H, Magi-Galluzzi C, Lu ZR (2015) EDB fibronectin specific peptide for prostate cancer targeting. Bioconjug Chem 26:830–838
Han Z, Li Y, Roelle S, Zhou Z, Liu Y, Sabatelle R, DeSanto A, Yu X, Zhu H, Magi-Galluzzi C, Lu ZR (2017) Targeted contrast agent specific to an oncoprotein in tumor microenvironment with the potential for detection and risk stratification of prostate cancer with MRI. Bioconjug Chem 28:1031–1040
Han Z, Cheng H, Parvani JG, Zhou Z, Lu ZR (2018) Magnetic resonance molecular imaging of metastatic breast cancer by targeting extradomain-B fibronectin in the tumor microenvironment. Magn Reson Med 79:3135–3143
Mandel U, Gaggero B, Reibel J et al (1994) Oncofetal fibronectins in oral carcinomas: correlation of two different types. APMIS 102:695–702
Gioanni J, Fischel JL, Lambert JC, Demard F, Mazeau C, Zanghellini E, Ettore F, Formento P, Chauvel P, Lalanne CM, Courdi A (1988) Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol 24:1445–1455
Rheinwald JG, Beckett MA (1981) Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res 41:1657–1663
Mirzayans R, Andrais B, Murray D (2018) Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers (Basel) 10:118
Amend SR, Torga G, Lin KC et al (2019) Polyploid giant cancer cells: unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate 79:1489–1497
Bharadwaj D, Mandal M (2019) Senescence in polyploid giant cancer cells: a road that leads to chemoresistance. Cytokine Growth Factor Rev 52:68–75
Momose F, Araida T, Negishi A, Ichijo H, Shioda S, Sasaki S (1989) Variant sublines with different metastatic potentials selected in nude mice from human oral squamous cell carcinomas. J Oral Pathol Med 18:391–395
Prince MR, Arnoldus C, Frisoli JK (1996) Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging 6:162–166
Kapoor C, Vaidya S, Wadhwan V, Malik S (2015) Lymph node metastasis: a bearing on prognosis in squamous cell carcinoma. Indian J Cancer 52:417–424
Li Y, Liu K, Ke Y, Zeng Y, Chen M, Li W, Liu W, Hua X, Li Z, Zhong Y, **e C, Yu H (2019) Risk factors analysis of pathologically confirmed cervical lymph nodes metastasis in oral squamous cell carcinoma patients with clinically negative cervical lymph node: results from a cancer center of Central China. J Cancer 10:3062–3069
Zbären P, Nuyens M, Caversaccio M, Stauffer E (2006) Elective neck dissection for carcinomas of the oral cavity: occult metastases, neck recurrences, and adjuvant treatment of pathologically positive necks. Am J Surg 191:756–760
Dhawan I, Sandhu SV, Bhandari R, Sood N, Bhullar RK, Sethi N (2016) Detection of cervical lymph node micrometastasis and isolated tumor cells in oral squamous cell carcinoma using immunohistochemistry and serial sectioning. J Oral Maxillofac Pathol 20:436–444
D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A, Kane S, Arya S, Ghosh-Laskar S, Chaturvedi P, Pai P, Nair S, Nair D, Badwe R, Head and Neck Disease Management Group (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373:521–529
Zhou Z, Qutaish M, Han Z (2015) MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun 6:7984
Harisi R, Jeney A (2015) Extracellular matrix as target for antitumor therapy. Onco Targets Ther 8:1387–1398
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR (2019) Targeting tumor microenvironment for cancer therapy. Int J Mol Sci 20:840
Raavé R, van Kuppevelt TH, Daamen WF (2018) Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release 274:1–8
Chang JH, Wu CC, Yuan KSP, Wu ATH, Wu SY (2017) Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget 8:55600–55612
Brands MT, Smeekens EAJ, Takes RP (2019) Time patterns of recurrence and second primary tumors in a large cohort of patients treated for oral cavity cancer. Cancer Med 8:5810–5819
Horshtuis K, Nederveen AJ, de Feiter MW et al (2009) Map** of T1-values and gadolinium-concentrations in MRI as indicator of disease activity in luminal Crohn’s disease: a feasibility study. J Magn Reson Imaging 29:488–493
de Bree R, Takes RP, Shah JP, Hamoir M, Kowalski LP, Robbins KT, Rodrigo JP, Sanabria A, Medina JE, Rinaldo A, Shaha AR, Silver C, Suárez C, Bernal-Sprekelsen M, Ferlito A (2019) Elective neck dissection in oral squamous cell carcinoma: past, present and future. Oral Oncol 90:87–93
Acknowledgments
ZR Lu is M. Frank Rudy and Margaret Domiter Rudy Professor of Biomedical Engineering. We also wish to thank Adam Kresak and Jenifer Mikulan at the Case Comprehensive Cancer Center for their technical assistance on the project.
Funding
This work was supported in part by the National Institutes of Health grants R01 CA194518, R01211762, and R44 CA199826, and by the Tissue Resources Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30 CA43703).
Author information
Authors and Affiliations
Contributions
Conceptual design of the research was coordinated by ZRL and RCH. All aspects of experimental execution were done by RCH. NRA and PLQ contributed to MRI data acquisition. NRA and AMV contributed to in vitro and in vivo data interpretation. IS reviewed all histological slides. AA assisted on clinical aspects of oral cancer, and DM assisted regarding MRI technique. The manuscript was prepared by RCH and ZRL and critically reviewed by NRA, PLQ, AMV, DM, AA, and IS.
Corresponding author
Ethics declarations
Conflict of Interest
ZRL is a co-founder of Molecular Theranostics, LLC, which is focused on the commercialization of targeted MRI contrast agents.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1503 kb)
Rights and permissions
About this article
Cite this article
Hall, R.C., Ayat, N.R., Qiao, P.L. et al. Preclinical Assessment of the Effectiveness of Magnetic Resonance Molecular Imaging of Extradomain-B Fibronectin for Detection and Characterization of Oral Cancer. Mol Imaging Biol 22, 1532–1542 (2020). https://doi.org/10.1007/s11307-020-01524-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01524-6