Abstract
Introduction
The impact of maternal coronavirus disease 2019 (COVID-19) infection on fetal health remains to be precisely characterized.
Objectives
Using metabolomic profiling of newborn umbilical cord blood, we aimed to investigate the potential fetal biological consequences of maternal COVID-19 infection.
Methods
Cord blood plasma samples from 23 mild COVID-19 cases (mother infected/newborn negative) and 23 gestational age-matched controls were analyzed using nuclear magnetic spectroscopy and liquid chromatography coupled with mass spectrometry. Metabolite set enrichment analysis (MSEA) was used to evaluate altered biochemical pathways due to COVID-19 intrauterine exposure. Logistic regression models were developed using metabolites to predict intrauterine exposure.
Results
Significant concentration differences between groups (p-value < 0.05) were observed in 19 metabolites. Elevated levels of glucocorticoids, pyruvate, lactate, purine metabolites, phenylalanine, and branched-chain amino acids of valine and isoleucine were discovered in cases while ceramide subclasses were decreased. The top metabolite model including cortisol and ceramide (d18:1/23:0) achieved an Area under the Receiver Operating Characteristics curve (95% CI) = 0.841 (0.725–0.957) for detecting fetal exposure to maternal COVID-19 infection. MSEA highlighted steroidogenesis, pyruvate metabolism, gluconeogenesis, and the Warburg effect as the major perturbed metabolic pathways (p-value < 0.05). These changes indicate fetal increased oxidative metabolism, hyperinsulinemia, and inflammatory response.
Conclusion
We present fetal biochemical changes related to intrauterine inflammation and altered energy metabolism in cases of mild maternal COVID-19 infection despite the absence of viral infection. Elucidation of the long-term consequences of these findings is imperative considering the large number of exposures in the population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) infection has affected over 260 million people and led to 5 million deaths worldwide (WHO COVID-19 Dashboard. Geneva: World Health Organization (WHO, 2019). The SARS-CoV-2 virus triggers a complex interaction between the host and the virus, leading to the reprogramming of the host’s metabolism (Thaker et al., 2019). In pregnancy, COVID-19 infection can result in a spectrum from asymptomatic to critical disease (Lokken et al., 2021). Although most of maternal COVID-19 infections are asymptomatic or cause mild disease, pregnant women are more susceptible to SARS-CoV-2 infection and are more likely to require intensive care unit care than nonpregnant adults (Ellington et al., 2020). Given the complex maternal physiologic and immune function changes during pregnancy, the interaction between the maternal immune response to the virus and the effects of COVID-19 infection on the fetus and neonate remains uncertain (Saadaoui et al., 2021).
Metabolomics is a discipline that aims to globally investigate small molecule metabolites and their interactions within a biological system (German et al., 2005). Due to the high-throughput characterization and interpretation of metabolites, metabolomics is a sensitive predictor of phenotype and rapidly reflects cellular perturbations. Recently, metabolomics was characterized as the ‘stethoscope’ of the twenty-first century because of its significant potential role in clinical medicine (Ashrafian et al., 2021). As metabolites show a wide range of both chemical properties and concentrations, therefore, there is no single instrument capable of simultaneously measuring the whole metabolome. Both targeted and untargeted approaches are utilized; the major platforms include Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy and mass spectrometry (MS) coupled with liquid chromatography (Wishart, 2011). Accumulating metabolomic literature report perturbations of biochemical pathways with remarkable alterations in the lipid and amino acid profiles in the non-pregnant COVID-19 infected population (Mussap & Fanos, 2021).
There is growing data on vertical transmission and neonatal outcomes of maternal COVID-19 infection (Norman et al., 2021; Saadaoui et al., 2021). Despite the evidence suggesting low risk of vertical transmission of SARS-CoV-2, population-based data indicates that maternal SARS-CoV-2 infection is associated with greater rates of neonatal co-morbidities (Norman et al., 2021). In addition, the effects of SARS-CoV-2 infection to the placenta is currently poorly understood, and the mechanisms of neonatal effects remain uncertain (Wastnedge et al., 2021). Cord blood is the best reflector of the fetal wellbeing based on its role in controlling the availability of nutrients and oxygen to the fetus. To the authors’ knowledge, metabolomics has never been utilized in the evaluation of fetal effects of maternal COVID-19 using cord blood. For these reasons, we performed a targeted multi-platform metabolomic analysis on cord blood. A combination of 1H NMR and direct injection coupled with liquid chromatography-tandem MS (DI-LC-MS/MS) based metabolomic analysis was performed. Using this comprehensive data set, we aimed to systematically evaluate fetal metabolic perturbations associated with mild maternal COVID-19 disease in the absence of fetal transmission. Mild disease is the most common clinical presentation. Secondly, we attempted to identify metabolomics markers that can be used for the detection of fetal exposure to COVID-19.
2 Materials and methods
2.1 Study population and sample collection
The study consisted of 23 pregnant women with confirmed mild COVID-19 infection at delivery and 23 age-matched uncomplicated term pregnancies. Institutional review board (IRB) approval was provided by Beaumont Hospital (approval #2020-186). All newborns tested negative for the SARS-CoV-2 virus. Inclusion criteria included singleton gestation delivered at term (> 37 weeks) at Beaumont Hospital, Royal Oak, Michigan, U.S.A., and pregnancies with mild symptoms of COVID-19 with maternal age ≥ 18 years. Exclusion criteria included severe or critical disease, steroid treatment, maternal conditions requiring chronic immunosuppression, previous COVID-19 vaccination, preterm birth, multifetal pregnancies, and the presence of other infections.
The diagnosis of COVID-19 was made by real-time reverse transcription-polymerase chain reaction (RT-PCR) assay performed on nasopharyngeal swab samples. Following written patient consent, cord blood samples were collected within a few minutes of delivery. This included double clam** of the umbilical cord and subsequent cutting of the cord. Subsequently, the 3′′- 4′′ area of the umbilical cord was cleansed with a sterile swab to remove any maternal blood and contaminants. Collection of blood was performed prior to the delivery of the placenta which additionally helps to avoid maternal blood contamination. A sterile butterfly needle or syringe was subsequently used for venipuncture and blood was transferred to vacutainer tubes. Umbilical vein blood was used for sampling. Blood specimens were expeditiously transferred to the laboratory in EDTA tubes and whole blood centrifugation was initiated within 30 min following collection at 4000 g for 10 min at 4 °C. The plasma was collected, divided into 250 µl size aliquots, and stored at − 80 °C for analysis. All specimens were frozen within one hour of collection, thawed once, and were used only for the metabolomic analyses presented herein.
2.2 1H NMR-based metabolomic analysis
1H NMR spectroscopic analysis of plasma specimens was carried out as follows; samples were prepared using a method described by Mercier et al. (2011). A detailed description of sample preparation for 1H NMR analysis is presented in the Supplementary Methods section. Following sample preparation, non-destructive NMR measurements for all plasma samples were conducted on Bruker Ascend HD NMR spectrometer equipped with a TCI probe operating at a magnetic field strength of 14.1 T and at a nominal proton frequency of 600.13 MHz. Spectra were acquired using a randomized running order at 25 °C using Bruker Topspin 3.1 software. Two hundred and fifty-six transients were acquired and 1H chemical shifts were calibrated using a singlet produced by the methyl groups of DSS (set to 0 ppm, 500 μM). All 1H NMR spectra were processed and profiled using Bayesil (Ravanbakhsh et al., 2015).
2.3 Direct injection liquid chromatography coupled with mass spectrometry (DI_UPLC-MS/MS)
The chemicals, reagents, plasma sample preparation, and DI-UPLC-MS/MS analysis are discussed in detail in the Supplementary Methods section. In brief, plasma extracts were analyzed using an Exion liquid chromatography unit coupled with a Quadrupole Trap (QTRAP) 6500 + mass spectrometer (AB SCIEX LLC; Redwood City, CA, USA). Plasma sample extracts were separated using the MxP Quant 500 C18 column with an attached guard and pre-column mixer (Biocrates Life Sciences, AG, Innsbruck, Austria). For comprehensive metabolomics and lipid analysis, Quant 500 kit used direct flow injections (FIA). This kit allows coverage of up to 630 metabolites from 26 biochemical classes, with advanced and reproducible metabolomics technology for the quantification of a broad range of metabolites. All data were extracted using the MetIDQ software following Biocrates instructions (Biocrates, Innsbruck, Austria).
2.4 Statistical analysis
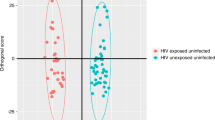
Using MetaboAnalyst (v 5.0) (Pang et al., 1A displays the results of the PLS-DA analysis. Good separation was observed between the two groups; however, following tenfold cross-validation (permutation testing using 2000 repeats) the model did not achieve statistical significance (Supplementary Fig. 1) (p = 0.392). Failure to achieve statistical significance is very likely due to the relatively small number of cases in the study. The VIP plot ranking metabolites for their power to discriminate COVID-19 cases from controls is shown in Fig. 1B. Cortisol, lactate, and cortisone appeared to be the best predictors of COVID-19 cases.
Table 2 presents logistic regression models for the detection of COVID-19 based on the top metabolites. These biomarker models are developed for the prediction of fetal exposure to maternal COVID-19 and potentially to identify those newborns who may potentially have short- and long-term adverse outcomes. The best diagnostic model combined cortisol and ceramide (d18:1/23:0) concentrations and achieved an AUC (95% CI) = 0.841 (0.725–0.957) with a sensitivity of 91.7% and specificity of 69.1%. The second metabolite model including ceramide (d18:1/23:0), triglyceride (22:5_34:1), and hypoxanthine performed well with an AUC (95% CI) = 0.765 (0.609–0.922) and a sensitivity of 83.3% and specificity of 81.8%. The logistic regression model equations including Z-values and odd ratios are presented in the supplementary results section.
MSEA revealed multiple metabolic pathways that were significantly perturbed in cord blood collected from maternal COVID-19 cases. Table 3 presents each perturbed pathway with the number of metabolite hits, enrichment impact, and p-values. The column “total” represents the total number of known metabolites involved in a particular metabolic pathway and the column “hits” indicates the number of metabolites in said pathway in patients with COVID-19. Pathways with the highest number of hits along with significant p-values have the greatest enrichment impact.
Figure 2 displays the results of the fold enrichment analysis based on the p-value and the enrichment contribution. The intensity of the red color indicates how significantly (p-value) the individual pathway has been perturbed due to the disease, while the length of the bar represents the enrichment contribution. The four most significantly affected pathways in COVID-19 cases were steroidogenesis, pyruvate metabolism, gluconeogenesis, and the Warburg effect.
Figure 3 presents the global network change using significantly altered metabolites (p < 0.05). These include altered steroidogenesis, gluconeogenesis, purine metabolism, sphingolipid-ceramide, and tyrosine/branched-chain amino acid network.
4 Discussion
Human SARS-CoV-2 infection precipitates a complex interaction between the metabolisms of the host and virus, forming the immune response and a wide range of outcomes, from asymptomatic to critical disease. Metabolomics has identified significant biochemical changes and predicted disease progression in patients with COVID-19 (Mussap & Fanos, 2021). In our study, we present significant changes in the fetal metabolic profile using cord blood that exhibited good separation between maternal COVID-19 cases and controls. We found significant alterations in the steroidogenesis and gluconeogenesis pathways with fetal exposure to maternal infection, in the absence of fetal infection. Further, cord blood metabolites displayed good predictive accuracy for the prediction of intrauterine exposure to maternal COVID-19 infection. A two metabolite achieved an AUC (95% CI) = 0.841 (0.725–0.957). These results indicate a fetal response to an inflammatory maternal environment even in the absence of demonstrable viral infection.
Accumulating data suggests a low risk for vertical transmission of the COVID-19 virus (Yuan et al., 2021), however, adverse perinatal outcomes have been reported, including pre-eclampsia and fetal growth restriction (Rosenbloom et al., 2021). Maternal immune activation and inflammation in response to viral infection have been linked to a spectrum of adverse short- and long-term effects in infants (Leon-Juarez et al., 2017). Maternal response to viral infection induces the production of proinflammatory cytokines, including interleukin-6 and TNF alpha which can cross the placental barrier (Ashdown et al., 2006). Placental histology in COVID-19 pregnancies, primarily of uninfected fetuses, reveal chronic inflammatory changes including lymphohistiocytic villitis, chronic histiocytic intervillositis, and chronic deciduitis (Wong et al., 2021). Altered metabolic changes in cord blood have been previously presented in the setting of placental inflammation (Chen et al., 2020). We were therefore interested in determining whether there were any safety signals in the uninfected but exposed fetus. In our study, hypoxanthine and xanthine were upregulated indicating upregulation of purine metabolism which has previously been shown to occur in the setting of intrauterine inflammation (Brown et al., 2017; Esther et al., 2008). We also present altered tyrosine and branched-chain amino acid network in COVID-19 cases. Accumulation of phenylalanine during inflammation and in COVID-19 patients has been previously reported due to downregulation of the conversion of phenylalanine to tyrosine (Kimhofer et al., 2020; Mussap & Fanos, 2021). Branched‐chain amino acids are known to promote endothelial dysfunction via increased reactive oxygen species generation and inflammation (Zhenyukh et al., 2018). Additionally, we identified decreased levels of several ceramide subclasses in the cord blood of COVID-19 cases. Ceramides belong to the sphingolipid family. These are essential constituents of cell organelles and membranes that play a role in signal transduction, cell growth, differentiation, proliferation, migration, apoptosis, and death. Down-regulation of ceramides has been presented in fetal placental vasculature of preeclampsia patients (Del Gaudio et al., 2020). Interestingly however, ceramides have been shown to reflect the progression of respiratory distress in adult COVID-19 patients (Khodadoust, 2021), and the neutralization or degradation of ceramides on the cell surface has been shown to protect against infection (Kornhuber et al., 2021). The depleted levels of ceramides in fetal cord blood in maternal COVID-19 infection may reflect an attempt to encounter inflammation. Further investigation is warranted.
We identified dysregulation of several fetal metabolomic pathways. These include steroidogenesis, pyruvate metabolism, gluconeogenesis, and the Warburg effect. Steroidogenesis was the most perturbed biochemical pathway and manifested by elevated levels of cortisol and cortisone (Fig. 3). Glucocorticoids play a key role in regulating fetal intrauterine growth, affecting fetal metabolism and homeostasis (Braun et al., 2013). It is also known that glucocorticoids lead to increased gluconeogenesis and energy metabolism (Khani & Tayek, 2001), and that cord blood cortisol is a marker for placental–fetal hypothalamic–pituitary–adrenal (HPA) axis activation (Gravett et al., 2000). Elevated in-utero cortisol levels were previously reported in Zika virus animal models (Trus et al., 2018). Fetuses and preterm newborns exposed to chronic intrauterine infections were previously reported to have elevated amniotic fluid and cord blood cortisol levels (Farr et al., 1980; Manabe et al., 2005). Further, proinflammatory cytokines inhibit human placental 11 beta-hydroxysteroid dehydrogenase type 2 (11β-HSD 2), which is an enzyme that converts cortisol to cortisone. This enzyme functions as a protective mechanism against excessive maternal cortisol, and the downregulation of this enzyme results in increased fetal cortisol levels (Kossintseva et al., 2006). The downregulation of 11β-HSD 2 was previously shown in maternal HIV and preeclampsia patients (Shallie et al., 2020). Our results indicate a more significant elevation in cortisol levels relative to cortisone suggesting similar changes in COVID-19 infected mothers. Hence, our findings suggest the need for investigating any impact of intrauterine exposure and evaluating the placental-fetal HPA axis even in the absence of vertical transmission. In addition to steroidogenesis, other top altered metabolomic pathways including pyruvate metabolism, gluconeogenesis, and the Warburg effect indicate altered fetal energy metabolism. Previously, these cord blood metabolomic changes were linked to newborn hyperinsulinemia (Kadakia et al., 2018). Additionally, elevated cord blood levels of branched-chain amino acids are known to associate with the development of diabetes (Zhenyukh et al., 2018). Centers for Disease Control and Prevention also recently reported an increased risk of diabetes among pediatric patients following SARS-COV-2 infection (Barrett et al., 2022). Further validation is required to explore the altered fetal energy metabolism and long-term pediatric consequences of maternal COVID-19. In addition to pathway analysis, we sought to identify metabolite biomarkers of fetal exposure. We reasoned that this might be important for longitudinally tracking newborns for persistence of these metabolic perturbations. The biomarker algorithm displayed good predictive accuracy based on the AUC values. It would be important to determine whether these alterations are transient and resolve after removal from the intrauterine environment.
This study is not without limitations. First, our sample size was modest making it difficult to evaluate the performance of the models in a separate independent validation group. However, we have ensured the generalizability of our results by performing stringent tenfold cross-validation. Additionally, we only included COVID-19 patients with mild symptoms which warrants further evaluation of the fetal metabolic profile in cases with severe or critical disease. Further, one other limitation is that the MSEA analysis uses p-values to represent the significance of each altered pathway instead of more stringent criteria, such as Bonferroni testing scores. Although more stringent multiple comparisons are important, they can lead to lead to a high rate of false negatives. Contrastingly, our study has several strengths. Since most pregnant women fall in the mild category and have mild symptoms, our findings are relevant to the real-world situation. Additionally, by including only mild cases, we limited the confounding effect of varying disease severity on the metabolomic profile. Further, including only term pregnancies excluded metabolomic changes related to prematurity as a potential confounder. Our finding of elevated cord cortisol levels in COVID-19 cases was previously shown to be affected by the delivery method and increase following vaginal delivery (Reissland & Wandinger, 1999). We also overcame this confounding factor by including similar rates of cesarean section births between cases and controls (Table 1). Our approach of combining 1H NMR and DI-LC-MS/MS (Quant 500 kit technology) represents one of the most comprehensive targeted metabolomics analyses as it evaluates more than 500 metabolites including many lipids. Finally, this is the first metabolomics study to show altered biochemical pathways of cord blood collected from COVID-19 cases.
This study adds new findings to our accumulating knowledge on the potential fetal effects of maternal COVID-19 disease. We propose that the next step involve the use of larger case numbers along with an investigation of moderate and severe maternal coronavirus disease. As our findings get validated in future studies, longitudinal surveillance of the affected infants will be warranted given the known impact of intrauterine inflammation on neurodevelopmental disorders.
References
Ashdown, H., Dumont, Y., Ng, M., Poole, S., Boksa, P., & Luheshi, G. N. (2006). The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Molecular Psychiatry, 11, 47–55.
Ashrafian, H., Sounderajah, V., Glen, R., Ebbels, T., Blaise, B. J., Kalra, D., Kultima, K., Spjuth, O., Tenori, L., Salek, R. M., Kale, N., Haug, K., Schober, D., Rocca-Serra, P., O’Donovan, C., Steinbeck, C., Cano, I., de Atauri, P., & Cascante, M. (2021). Metabolomics: The stethoscope for the twenty-first century. Medical Principles and Practice, 30, 301–310.
Barrett, C. E., Koyama, A. K., Alvarez, P., Chow, W., Lundeen, E. A., Perrine, C. G., Pavkov, M. E., Rolka, D. B., Wiltz, J. L., Bull-Otterson, L., Gray, S., Boehmer, T. K., Gundlapalli, A. V., Siegel, D. A., Kompaniyets, L., Goodman, A. B., Mahon, B. E., Tauxe, R. V., Remley, K., & Saydah, S. (2022). Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years—United States, March 1, 2020–June 28, 2021. MMWR. Morbidity and Mortality Weekly Report, 71, 59–65.
Braun, T., Challis, J. R., Newnham, J. P., & Sloboda, D. M. (2013). Early-life glucocorticoid exposure: The hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocrine Reviews, 34, 885–916.
Brown, A. G., Tulina, N. M., Barila, G. O., Hester, M. S., & Elovitz, M. A. (2017). Exposure to intrauterine inflammation alters metabolomic profiles in the amniotic fluid, fetal and neonatal brain in the mouse. PLoS ONE, 12, e0186656.
Chen, Q., Gouilly, J., Ferrat, Y. J., Espino, A., Glaziou, Q., Cartron, G., El Costa, H., Al-Daccak, R., & Jabrane-Ferrat, N. (2020). Metabolic reprogramming by Zika virus provokes inflammation in human placenta. Nature Communications, 11, 2967.
Del Gaudio, I., Sasset, L., Lorenzo, A. D., & Wadsack, C. (2020). Sphingolipid signature of human feto-placental vasculature in preeclampsia. International Journal of Molecular Science, 21, 1019.
Ellington, S., Strid, P., Tong, V. T., Woodworth, K., Galang, R. R., Zambrano, L. D., Nahabedian, J., Anderson, K., & Gilboa, S. M. (2020). Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR. Morbidity and Mortality Weekly Report, 69, 769–775.
Esther, C. R., Jr., Alexis, N. E., Clas, M. L., Lazarowski, E. R., Donaldson, S. H., Ribeiro, C. M., Moore, C. G., Davis, S. D., & Boucher, R. C. (2008). Extracellular purines are biomarkers of neutrophilic airway inflammation. European Respiratory Journal, 31, 949–956.
Farr, A. G., Cho, Y., & De Bruyn, P. P. (1980). The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. The American Journal of Anatomy, 157, 265–284.
German, J. B., Hammock, B. D., & Watkins, S. M. (2005). Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics, 1, 3–9.
Gravett, M. G., Hitti, J., Hess, D. L., & Eschenbach, D. A. (2000). Intrauterine infection and preterm delivery: Evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. American Journal of Obstetrics and Gynecology, 182, 1404–1413.
Kadakia, R., Scholtens, D. M., Rouleau, G. W., Talbot, O., Ilkayeva, O. R., George, T., & Josefson, J. L. (2018). Cord blood metabolites associated with newborn adiposity and hyperinsulinemia. Journal of Pediatrics, 203(144–149), e1.
Karnovsky, A., Weymouth, T., Hull, T., Tarcea, V. G., Scardoni, G., Laudanna, C., Sartor, M. A., Stringer, K. A., Jagadish, H. V., & Burant, C. (2012). Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics, 28, 373–380.
Khani, S., & Tayek, J. A. (2001). Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clinical Science (london, England), 101, 739–747.
Khodadoust, M. M. (2021). Inferring a causal relationship between ceramide levels and COVID-19 respiratory distress. Science and Reports, 11, 20866.
Kimhofer, T., Lodge, S., Whiley, L., Gray, N., Loo, R. L., Lawler, N. G., Nitschke, P., Bong, S. H., Morrison, D. L., Begum, S., Richards, T., Yeap, B. B., Smith, C., Smith, K. G. C., Holmes, E., & Nicholson, J. K. (2020). Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS-CoV-2 infection. Journal of Proteome Research, 19, 4442–4454.
Kornhuber, J., Hoertel, N., & Gulbins, E. (2021). The acid sphingomyelinase/ceramide system in COVID-19. Molecular Psychiatry, 27, 307.
Kossintseva, I., Wong, S., Johnstone, E., Guilbert, L., Olson, D. M., & Mitchell, B. F. (2006). Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. American Journal of Physiology. Endocrinology and Metabolism, 290, E282–E288.
Leon-Juarez, M., Martinez-Castillo, M., Gonzalez-Garcia, L. D., Helguera-Repetto, A. C., Zaga-Clavellina, V., Garcia-Cordero, J., Flores-Pliego, A., Herrera-Salazar, A., Vazquez-Martinez, E. R., & Reyes-Munoz, E. (2017). Cellular and molecular mechanisms of viral infection in the human placenta. Pathogens and Disease. https://doi.org/10.1093/femspd/ftx093
Lokken, E. M., Huebner, E. M., Taylor, G. G., Hendrickson, S., Vanderhoeven, J., Kachikis, A., Coler, B., Walker, C. L., Sheng, J. S., Al-Haddad, B. J. S., McCartney, S. A., Kretzer, N. M., Resnick, R., Barnhart, N., Schulte, V., Bergam, B., Ma, K. K., Albright, C., Larios, V., … Adams Waldorf, K. M. (2021). Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. American Journal of Obstetrics and Gynecology, 225, 77.e1-77.e14.
Manabe, M., Nishida, T., Imai, T., Kusaka, T., Kawada, K., Okada, H., Okubo, K., Isobe, K., & Itoh, S. (2005). Cortisol levels in umbilical vein and umbilical artery with or without antenatal corticosteroids. Pediatrics International, 47, 60–63.
Mussap, M., & Fanos, V. (2021). Could metabolomics drive the fate of COVID-19 pandemic? A narrative review on lights and shadows. Clinical Chemistry and Laboratory Medicine, 59, 1891–1905.
Norman, M., Naver, L., Soderling, J., Ahlberg, M., Hervius Askling, H., Aronsson, B., Bystrom, E., Jonsson, J., Sengpiel, V., Ludvigsson, J. F., Hakansson, S., & Stephansson, O. (2021). Association of Maternal SARS-CoV-2 Infection in Pregnancy With Neonatal Outcomes. JAMA, 325, 2076–2086.
Pang, Z., Chong, J., Zhou, G., de Lima Morais, D. A., Chang, L., Barrette, M., Gauthier, C., Jacques, P. E., Li, S., & **a, J. (2021). MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Research, 49, W388–W396.
Ravanbakhsh, S., Liu, P., Bjordahl, T. C., Mandal, R., Grant, J. R., Wilson, M., Eisner, R., Sinelnikov, I., Hu, X., Luchinat, C., Greiner, R., & Wishart, D. S. (2015). Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE, 10, e0124219.
Reissland, P., & Wandinger, K. P. (1999). Increased cortisol levels in human umbilical cord blood inhibit interferon alpha production of neonates. Immunobiology, 200, 227–233.
Rosenbloom, J. I., Raghuraman, N., Carter, E. B., & Kelly, J. C. (2021). Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. American Journal of Obstetrics and Gynecology, 224, 623–624.
Saadaoui, M., Kumar, M., & Al Khodor, S. (2021). COVID-19 infection during pregnancy: Risk of vertical transmission, fetal, and neonatal outcomes. Journal of Personalized Medicine, 11, 483.
Shallie, P. D., Margolis, D., Shallie, O. F., & Naicker, T. (2020). Placental 11beta-HSD2 downregulated in HIV associated preeclampsia. Journal of Reproductive Immunology, 142, 103185.
Thaker, S. K., Ch’ng, J., & Christofk, H. R. (2019). Viral hijacking of cellular metabolism. BMC Biology, 17, 59.
Trus, I., Darbellay, J., Huang, Y., Gilmour, M., Safronetz, D., Gerdts, V., & Karniychuk, U. (2018). Persistent Zika virus infection in porcine conceptuses is associated with elevated in utero cortisol levels. Virulence, 9, 1338–1343.
Wastnedge, E. A. N., Reynolds, R. M., van Boeckel, S. R., Stock, S. J., Denison, F. C., Maybin, J. A., & Critchley, H. O. D. (2021). Pregnancy and COVID-19. Physiological Reviews, 101, 303–318.
WHO. (2019). WHO COVID-19 dashboard. World Health Organization.
Wishart, D. S. (2011). Advances in metabolite identification. Bioanalysis, 3, 1769–1782.
Wong, Y. P., Khong, T. Y., & Tan, G. C. (2021). The effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics (basel), 11, 94.
**a, J., Mandal, R., Sinelnikov, I. V., Broadhurst, D., & Wishart, D. S. (2012). MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Research, 40, W127–W133.
**a, J., Psychogios, N., Young, N., & Wishart, D. S. (2009). MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Research, 37, W652–W660.
Yuan, J., Qian, H., Cao, S., Dong, B., Yan, X., Luo, S., Zhou, M., Zhou, S., Ning, B., & Zhao, L. (2021). Is there possibility of vertical transmission of COVID-19: A systematic review. Translational Pediatrics, 10, 423–434.
Zhenyukh, O., Gonzalez-Amor, M., Rodrigues-Diez, R. R., Esteban, V., Ruiz-Ortega, M., Salaices, M., Mas, S., Briones, A. M., & Egido, J. (2018). Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. Journal of Cellular and Molecular Medicine, 22, 4948–4962.
Author information
Authors and Affiliations
Contributions
OT completed project design, wrote the main text and analyzed the metabolomics data SS and SS collected specimens JI completed literature review and data collection AY, NA and SFG supervised the metabolomics experiment KW and AA designed the project, supervised patient diagnosis and sample collection RBS mentorship for manuscript writing, data interpretation.
Corresponding author
Ethics declarations
Competing interest
S.F.G. has received commercial support as a consultant from Biogen, Roche, Iollo and Coleman Research. The remaining authors report no conflicts of interest.
Research involving in human and animal participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turkoglu, O., Alhousseini, A., Sajja, S. et al. Fetal effects of mild maternal COVID-19 infection: metabolomic profiling of cord blood. Metabolomics 19, 41 (2023). https://doi.org/10.1007/s11306-023-01988-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-023-01988-x