Abstract
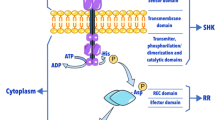
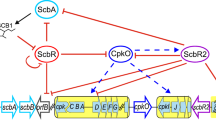
The two-component system (TCS) found in various organisms is a regulatory system, which is involved in the response by the organism to stimuli, thereby regulating the internal behavior of the cell. It is commonly found in prokaryotes and is an important signaling system in bacteria. TCSs are involved in the regulation of physiological and morphological differentiation of the industrially important microbes from the genus Streptomyces, which produce a vast array of bioactive secondary metabolites (SMs). Genetic engineering of TCSs can substantially increase the yield of target SMs, which is valuable for industrial-scale production. Research on TCS has mainly been completed in the model strain Streptomyces coelicolor. In this review, we summarize the recent advances in the functional identification and elucidation of the regulatory mechanisms of various TCSs in S. coelicolor, with a focus on their roles in the biosynthesis of important SMs.




Similar content being viewed by others
References
Aceti DJ, Champness WC (1998) Transcriptional regulation of Streptomyces coelicolor pathway-specific antibiotic regulators by the absA and absB loci. J Bacteriol 180:3100–3106
Ainsa JA, Parry HD, Chater KF (1999) A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol Microbiol 34:607–619
Allenby NEE, Laing E, Bucca G, Kierzek AM, Smith CP (2012) Diverse control of metabolism and other cellular processes in Streptomyces coelicolor by the PhoP transcription factor: genome-wide identification of in vivo targets. Nucleic Acids Res 40:9543–9556
Amin R, Reuther J, Bera A, Wohlleben W, Mast Y (2012) A novel GlnR target gene, nnaR, is involved in nitrate/nitrite assimilation in Streptomyces coelicolor. Microbiology 158:1172–1182
Amin R, Franz-Wachtel M, Tiffert Y, Heberer M, Meky M, Ahmed Y, Matthews A, Krysenko S, Jakobi M, Hinder M, Moore J, Okoniewski N, Macek B, Wohlleben W, Bera A (2016) Post-translational serine/threonine phosphorylation and lysine acetylation: a novel regulatory aspect of the global nitrogen response regulator GlnR in S. coelicolor M145. Front Mol Biosci 3:38
Anderson T, Brian P, Riggle P, Kong R, Champness W (1999) Genetic suppression analysis of non-antibiotic-producing mutants of the Streptomyces coelicolor absA locus. Microbiol (Reading 145:2343–2353
Anderson TB, Brian P, Champness WC (2001) Genetic and transcriptional analysis of absA, an antibiotic gene cluster-linked two-component system that regulates multiple antibiotics in Streptomyces coelicolor. Mol Microbiol 39:553–566
Antoraz S, Rico S, Rodriguez H, Sevillano L, Alzate JF, Santamaria RI, Diaz M (2017) The orphan response regulator Aor1 is a new relevant piece in the complex puzzle of Streptomyces coelicolor antibiotic regulatory network. Front Microbiol 8:2444
Apel AK, Sola-Landa A, Rodriguez-Garcia A, Martin JF (2007) Phosphate control of phoA, phoC and phoD gene expression in Streptomyces coelicolor reveals significant differences in binding of PhoP to their promoter regions. Microbiology 153:3527–3537
Arroyo-Perez EE, Gonzalez-Ceron G, Soberon-Chavez G, Georgellis D, Servin-Gonzalez L (2019) A novel two-component system, encoded by the sco5282/sco5283 genes, affects Streptomyces coelicolor morphology in liquid culture. Front Microbiol 10:1568
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Meier-Kolthoff JP, Klenk HP, Clement C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and Natural Products of Actinobacteria. Microbiol Mol Biol Rev 80:1–43
Barreiro C, Martinez-Castro M (2019) Regulation of the phosphate metabolism in Streptomyces genus: impact on the secondary metabolites. Appl Microbiol Biotechnol 103:1643–1658
Bednarz B, Kotowska M, Pawlik KJ (2019) Multi-level regulation of coelimycin synthesis in Streptomyces coelicolor A3(2). Appl Microbiol Biotechnol 103:6423–6434
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Bishop A, Fielding S, Dyson P, Herron P (2004) Systematic insertional mutagenesis of a streptomycete genome: a link between osmoadaptation and antibiotic production. Genome Res 14:893–900
Brian P, Riggle PJ, Santos RA, Champness WC (1996) Global negative regulation of Streptomyces coelicolor antibiotic synthesis mediated by an absa-encoded putative signal transduction system. J Bacteriol 178:3221–3231
Bush MJ, Tschowri N, Schlimpert S, Flardh K, Buttner MJ (2015) c-di-GMP signalling and the regulation of developmental transitions in streptomycetes. Nat Rev Microbiol 13:749–760
Butler MS (2008) Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep 25:475–516
Chang HM, Chen MY, Shieh YT, Bibb MJ, Chen CW (1996) The cutRS signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Mol Microbiol 21:1075–1085
Chen SS, Zheng GS, Zhu H, He HQ, Chen L, Zhang WW, Jiang WH, Lu YH (2016) Roles of two-component system AfsQ1/Q2 in regulating biosynthesis of the yellow-pigmented coelimycin P2 in Streptomyces coelicolor. Fed Eur Microbiol Soc Microbiol Lett 363:fnw160
Chen YL, Yang YP, Li GQ, Mao XF, Jia WD, Shi AP, Lu YH (2021) Investigation into the upstream signal transduction pathway of AfsQ1/Q2, a two-component regulatory system involved in regulation of antibiotic synthesis in Streptomyces coelicolor. Prog Biochem Biophys 48:450–464
de la Nieta RS, Antoraz S, Alzate JF, Santamaria RI, Diaz M (2020) Antibiotic production and antibiotic resistance: the two sides of AbrB1/B2, a two-component system of Streptomyces coelicolor. Front Microbiol 11:587750
Dutta R, Qin L, Inouye M (1999) Histidine kinases: diversity of domain organization. Mol Microbiol 34:633–640
Fernandez-Martinez LT, Santos-Beneit F, Martin JF (2012) Is PhoR-PhoP partner fidelity strict? PhoR is required for the activation of the pho regulon in Streptomyces coelicolor. Mol Genet Genomics 287:565–573
Fink D, Weissschuh N, Reuther J, Wohlleben W, Engels A (2002) Two transcriptional regulators GlnR and GlnRII are involved in regulation of nitrogen metabolism in Streptomyces coelicolor A3(2). Mol Microbiol 46:331–347
Fischer M, Falke D, Ronitz J, Haase A, Damelang T, Pawlik T, Sawers RG (2019) Hypoxia-induced synthesis of respiratory nitrate reductase 2 of Streptomyces coelicolor A3(2) depends on the histidine kinase OsdK in mycelium but not in spores. Microbiology 165:905–916
Gongerowska-Jac M, Szafran MJ, Mikolajczyk J, Szymczak J, Bartynska M, Gierlikowska A, Bialy S, Elliot MA, Jakimowicz D (2021) Global chromosome topology and the two-component systems in concerted manner regulate transcription in Streptomyces. Msystems 6:e0114221
Guthrie EP, Flaxman CS, White J, Hodgson DA, Bibb MJ, Chater KF (1998) A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiol (Reading) 144:727–738
He JM, Zhu H, Zheng GS, Liu PP, Wang J, Zhao GP, Zhu GQ, Jiang WH, Lu YH (2016) Direct involvement of the master nitrogen metabolism regulator GlnR in antibiotic biosynthesis in Streptomyces. J Biol Chem 291:26443–26454
Homerova D, Knirschova R, Kormanec J (2002) Response regulator ChiR regulates expression of chitinase gene, chiC, in Streptomyces coelicolor. Folia Microbiol 47:499–505
Hong HJ, Paget MSB, Buttner MJ (2002) A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Mol Microbiol 44:1199–1211
Hong HJ, Hutchings MI, Neu JM, Wright GD, Paget MSB, Buttner MJ (2004) Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol Microbiol 52:1107–1121
Hong HJ, Hutchings MI, Hill LM, Buttner MJ (2005) The role of the novel fem protein VanK in vancomycin resistance in Streptomyces coelicolor. J Biol Chem 280:13055–13061
Hong HJ, Hutchings MI, Buttner MJ (2008) Vancomycin resistance VanS/VanR two-component systems. Adv Exp Med Biol 631:200–213
Hong HJ (2016) Construction of a bioassay system to identify extracellular agents targeting bacterial cell envelope. Methods Mol Biol 1440:125–137
Honma S, Ito S, Yajima S, Sasaki Y (2021) Nitric oxide signaling for actinorhodin production in Streptomyces coelicolor A3(2) via the DevS/R two-component system. Appl Environ Microbiol 87:e0048021
Howell A, Dubrac S, Noone D, Varughese KI, Devine K (2006) Interactions between the YycFG and PhoPR two-component systems in Bacillus subtilis: the PhoR kinase phosphorylates the non-cognate YycF response regulator upon phosphate limitation. Mol Microbiol 59:1199–1215
Huang J, Lih CJ, Pan KH, Cohen SN (2001) Global analysis of growth phase responsive gene expression and regulation of antibiotic biosynthetic pathways in Streptomyces coelicolor using DNA microarrays. Genes Dev 15:3183–3192
Hutchings MI, Hoskisson PA, Chandra G, Buttner MJ (2004) Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiol (Reading) 150:2795–2806
Hutchings MI, Hong HJ, Buttner MJ (2006a) The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol Microbiol 59:923–935
Hutchings MI, Hong HJ, Leibovitz E, Sutcliffe IC, Buttner MJ (2006b) The σE cell envelope stress response of Streptomyces coelicolor is influenced by a novel lipoprotein, CseA. J Bacteriol 188:7222–7229
Hwang I, Chen HC, Sheen J (2002) Two-component signal transduction pathways in Arabidopsis. Plant Physiol 129:500–515
Ishizuka H, Horinouchi S, Kieser HM, Hopwood DA, Beppu T (1992) A putative two-component regulatory system involved in secondary metabolism in Streptomyces spp. J Bacteriol 174:7585–7594
Kilian R, Frasch HJ, Kulik A, Wohlleben W, Stegmann E (2016) The VanRS homologous two-component system VnlRSAb of the glycopeptide producer Amycolatopsis balhimycina activates transcription of the vanHAXSc genes in Streptomyces coelicolor, but not in A. balhimycina. Microb Drug Resist 22:499–509
Kim D-J, Forst S (2001) Genomic analysis of the histidine kinase family in bacteria and archaea. Microbiol (Reading) 147:1197–1212
Kim YJ, Moon AN, Song JY, Kim ES, Kim CJ, Chang YK (2009) Gene-expression analysis of acidic pH shock effects on two-component systems in Streptomyces coelicolor. Biotechnol Bioprocess Eng 14:584–590
Kormanec J, Sevcikova B, Homerova D (2000) Cloning of a two-component regulatory system probably involved in the regulation of chitinase in Streptomyces coelicolor A3(2). Folia Microbiol 45:397–406
Koteva K, Hong HJ, Wang XD, Nazi I, Hughes D, Naldrett MJ, Buttner MJ, Wright GD (2010) A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat Chem Biol 6:327–329
Lejeune C, Abreu S, Chaminade P, Dulermo T, David M, Werten S, Virolle MJ (2021) Impact of phosphate availability on membrane lipid content of the model strains, Streptomyces lividans and Streptomyces coelicolor. Front Microbiol 12:623919
Lewis RA, Wahab A, Bucca G, Laing EE, Moller-Levet CS, Kierzek A, Smith CP (2019) Genome-wide analysis of the role of the antibiotic biosynthesis regulator AbsA2 in Streptomyces coelicolor A3(2). PLoS ONE 14:e0200673
Li YQ, Chen PL, Chen SF, Wu D, Zheng J (2004) A pair of two-component regulatory genes ecrA1/A2 in S. coelicolor. J Zhejiang Univ Sci 5:173–179
Li L, Jiang WH, Lu YH (2017) A novel two-component system, GluR-GluK, involved in glutamate sensing and uptake in Streptomyces coelicolor. J Bacteriol 199:e0009717
Li L, Zhao YW, Ma JJ, Tao HN, Zheng GS, Chen J, Jiang WH, Lu YH (2020) The orphan histidine kinase PdtaS-p regulates both morphological differentiation and antibiotic biosynthesis together with the orphan response regulator PdtaR-p in Streptomyces. Microbiol Res 233:126411
Liu M, Zhang PP, Zhu YP, Lu T, Wang YM, Cao GX, Shi M, Chen XL, Tao MF, Pang XH (2019) Novel two-component system MacRS is a pleiotropic regulator that controls multiple morphogenic membrane protein genes in Streptomyces coelicolor. Appl Environ Microbiol 85:e0217818
Liu M, Xu WH, Zhu YP, Cui XQ, Pang XH (2021) The response regulator MacR and its potential in improvement of antibiotic production in Streptomyces coelicolor. Curr Microbiol 78:3696–3707
Lockey C, Edwards RJ, Roper DI, Dixon AM (2020) The extracellular domain of two-component system sensor kinase VanS from Streptomyces coelicolor binds vancomycin at a newly identified binding site. Sci Rep 10:1–13
Lu YH, Wang WH, Shu D, Zhang WW, Chen L, Qin ZJ, Yang S, Jiang WH (2007) Characterization of a novel two-component regulatory system involved in the regulation of both actinorhodin and a type I polyketide in Streptomyces coelicolor. Appl Microbiol Biotechnol 77:625–635
Lu YH, He JM, Zhu H, Yu ZY, Wang R, Chen YL, Dang FJ, Zhang WW, Yang S, Jiang WH (2011) An orphan histidine kinase, OhkA, regulates both secondary metabolism and morphological differentiation in Streptomyces coelicolor. J Bacteriol 193:3020–3032
Maciunas LJ, Porter N, Lee PJ, Gupta K, Loll PJ (2021) Structures of full-length VanR from Streptomyces coelicolor in both the inactive and activated states. Acta Crystallogr D Struct Biol 77:1027–1039
Martin JF, Sola-Landa A, Santos-Beneit F, Fernandez-Martinez LT, Prieto C, Rodriguez-Garcia A (2011) Cross-talk of global nutritional regulators in the control of primary and secondary metabolism in Streptomyces. Microb Biotechnol 4:165–174
Martin JF, Santos-Beneit F, Rodriguez-Garcia A, Sola-Landa A, Smith MCM, Ellingsen TE, Nieselt K, Burroughs NJ, Wellington EMH (2012) Transcriptomic studies of phosphate control of primary and secondary metabolism in Streptomyces coelicolor. Appl Microbiol Biotechnol 95:61–75
Martin JF, Rodriguez-Garcia A, Liras P (2017) The master regulator PhoP coordinates phosphate and nitrogen metabolism, respiration, cell differentiation and antibiotic biosynthesis: comparison in Streptomyces coelicolor and Streptomyces avermitilis. J Antibiot 70:534–541
Martin-Martin S, Rodriguez-Garcia A, Santos-Beneit F, Franco-Dominguez E, Sola-Landa A, Martin JF (2018) Self-control of the PHO regulon: the PhoP-dependent protein PhoU controls negatively expression of genes of PHO regulon in Streptomyces coelicolor. J Antibiot 71:113–122
Martinez-Castro M, Barreiro C, Martin JF (2018) Analysis and validation of the pho regulon in the tacrolimus-producer strain Streptomyces tsukubaensis: differences with the model organism Streptomyces coelicolor. Appl Microbiol Biotechnol 102:7029–7045
McKenzie NL, Nodwell JR (2007) Phosphorylated AbsA2 negatively regulates antibiotic production in Streptomyces coelicolor through interactions with pathway-specific regulatory gene promoters. J Bacteriol 189:5284–5292
McLean TC, Lo R, Tschowri N, Hoskisson PA, Al Bassam MM, Hutchings MI, Som NF (2019) Sensing and responding to diverse extracellular signals: an updated analysis of the sensor kinases and response regulators of Streptomyces species. Microbiology 165:929–952
Millan-Oropeza A, Henry C, Lejeune C, David M, Virolle MJ (2020) Expression of genes of the pho regulon is altered in Streptomyces coelicolor. Sci Rep 10:1–21
Moeker N, Brocker M, Schaffer S, Kraemer R, Morbach S, Bott M (2004) Deletion of the genes encoding the MtrA-MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoprotection. Mol Microbiol 54:420–438
Molle V, Buttner MJ (2000) Different alleles of the response regulator gene bldM arrest Streptomyces coelicolor development at distinct stages. Mol Microbiol 36:1265–1278
Nguyen KT, Willey JM, Nguyen LD, Nguyen LT, Viollier PH, Thompson CJ (2002) A central regulator of morphological differentiation in the multicellular bacterium Streptomyces coelicolor. Mol Microbiol 46:1223–1238
Novotna GB, Kwun MJ, Hong HJ (2016) In vivo characterization of the activation and interaction of the VanR-VanS two-component regulatory system controlling glycopeptide antibiotic resistance in two related Streptomyces species. Antimicrob Agents Chemother 60:1627–1637
O’Connor TJ, Kanellis P, Nodwell JR (2002) The ramC gene is required for morphogenesis in Streptomyces coelicolor and expressed in a cell type-specific manner under the direct control of RamR. Mol Microbiol 45:45–57
O’Connor TJ, Nodwell JR (2005) Pivotal roles for the receiver domain in the mechanism of action of the response regulator RamR of Streptomyces coelicolor. J Mol Biol 351:1030–1047
Paget MS, Leibovitz E, Buttner MJ (1999) A putative two-component signal transduction system regulates σE, a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Mol Microbiol 33:97–107
Parkinson JS, Kofoid EC (1992) Communication modules in bacterial signaling proteins. Annu Rev Genet 26:71–112
Pullan ST, Chandra G, Bibb MJ, Merrick M (2011) Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics 12:1–14
Rico S, Santamaria RI, Yepes A, Rodriguez H, Laing E, Bucca G, Smith CP, Diaz M (2014a) Deciphering the regulon of Streptomyces coelicolor AbrC3, a positive response regulator of antibiotic production. Appl Environ Microbiol 80:2417–2428
Rico S, Yepes A, Rodriguez H, Santamaria J, Antoraz S, Krause EM, Diaz M, Santamaria RI (2014b) Regulation of the AbrA1/A2 two-component system in Streptomyces coelicolor and the potential of its deletion strain as a heterologous host for antibiotic production. PLoS ONE 9:e109844
Rodriguez H, Rico S, Diaz M, Santamaria RI (2013) Two-component systems in Streptomyces: key regulators of antibiotic complex pathways. Microb Cell Fact 12:1–10
Rodriguez H, Rico S, Yepes A, Franco-Echevarria E, Antoraz S, Santamaria RI, Diaz M (2015) The two kinases, AbrC1 and AbrC2, of the atypical two-component system AbrC are needed to regulate antibiotic production and differentiation in Streptomyces coelicolor. Front Microbiol 6:450
Rodriguez-Garcia A, Barreiro C, Santos-Beneit F, Sola-Landa A, Martin JF (2007) Genome-wide transcriptomic and proteomic analysis of the primary response to phosphate limitation in Streptomyces coelicolor M145 and in a ∆phoP mutant. Proteomics 7:2410–2429
Rodriguez-Garcia A, Sola-Landa A, Apel K, Santos-Beneit F, Martin JF (2009) Phosphate control over nitrogen metabolism in Streptomyces coelicolor: direct and indirect negative control of glnR, glnA, glnII and amtB expression by the response regulator PhoP. Nucleic Acids Res 37:3230–3242
Rozas D, Gullon S, Mellado RP (2012) A novel two-component system involved in the transition to secondary metabolism in Streptomyces coelicolor. PLoS ONE 7:e31760
Ryding NJ, Anderson TB, Champness WC (2002) Regulation of the Streptomyces coelicolor calcium-dependent antibiotic by absA, encoding a cluster-linked two-component system. J Bacteriol 184:794–805
San Paolo S, Huang J, Cohen SN, Thompson CJ (2006) rag genes: novel components of the RamR regulon that trigger morphological differentiation in Streptomyces coelicolor. Mol Microbiol 61:1167–1186
Santos-Beneit F, Rodriguez-Garcia A, Apel AK, Martin JF (2009a) Phosphate and carbon source regulation of two PhoP-dependent glycerophosphodiester phosphodiesterase genes of Streptomyces coelicolor. Microbiology 155:1800–1811
Santos-Beneit F, Rodriguez-Garcia A, Sola-Landa A, Martin JF (2009b) Cross-talk between two global regulators in Streptomyces: PhoP and AfsR interact in the control of afsS, pstS and phoRP transcription. Mol Microbiol 72:53–68
Santos-Beneit F, Barriuso-Iglesias M, Fernandez-Martinez LT, Martinez-Castro M, Sola-Landa A, Rodriguez-Garcia A, Martin JF (2011) The RNA rolymerase omega factor RpoZ is regulated by PhoP and has an important role in antibiotic biosynthesis and morphological differentiation in Streptomyces coelicolor. Appl Environ Microbiol 77:7586–7594
Santos-Beneit F, Rodriguez-Garcia A, Martin JF (2012) Overlap** binding of PhoP and AfsR to the promoter region of glnR in Streptomyces coelicolor. Microbiol Res 167:532–535
Santos-Beneit F, Rodriguez-Garcia A, Martin JF (2013) Identification of different promoters in the absA1-absA2 two-component system, a negative regulator of antibiotic production in Streptomyces coelicolor. Mol Genet Genomics 288:39–48
Shao ZH, Deng WX, Li SY, He JM, Ren SX, Huang WR, Lu YH, Zhao GP, Cai ZM, Wang J (2015) GlnR-mediated regulation of ectABCD transcription expands the role of the GlnR regulon to osmotic stress management. J Bacteriol 197:3041–3047
Sheeler NL, MacMillan SV, Nodwell JR (2005) Biochemical activities of the absA two-component system of Streptomyces coelicolor. J Bacteriol 187:687–696
Shu D, Chen L, Wang WH, Yu ZY, Ren C, Zhang WW, Yang S, Lu YH, Jiang WH (2009) afsQ1-Q2-sigQ is a pleiotropic but conditionally required signal transduction system for both secondary metabolism and morphological development in Streptomyces coelicolor. Appl Microbiol Biotechnol 81:1149–1160
Sola-Landa A, Rodriguez-Garcia A, Franco-Dominguez E, Martin JF (2005) Binding of PhoP to promoters of phosphate-regulated genes in Streptomyces coelicolor: identification of PHO boxes. Mol Microbiol 56:1373–1385
Sola-Landa A, Rodriguez-Garci A, Apel AK, Martin JF (2008) Target genes and structure of the direct repeats in the DNA-binding sequences of the response regulator PhoP in Streptomyces coelicolor. Nucleic Acids Res 36:1358–1368
Sola-Landa A, Rodriguez-Garcia A, Amin R, Wohlleben W, Martin JF (2013) Competition between the GlnR and PhoP regulators for the glnA and amtB promoters in Streptomyces coelicolor. Nucleic Acids Res 41:1767–1782
Som NF, Heine D, Holmes N, Knowles F, Chandra G, Seipke RF, Hoskisson PA, Wilkinson B, Hutchings MI (2017) The MtrAB two-component system controls antibiotic production in Streptomyces coelicolor. Microbiol 163 A3(2):1415–1419
Thomason P, Kay R (2000) Eukaryotic signal transduction via histidine-aspartate phosphorelay. J Cell Sci 113:3141–3150
Tian YQ, Fowler K, Findlay K, Tan HR, Chater KF (2007) An unusual response regulator influences sporulation at early and late stages in Streptomyces coelicolor. J Bacteriol 189:2873–2885
Tiffert Y, Supra P, Wurm R, Wohlleben W, Wagner R, Reuther J (2008) The Streptomyces coelicolor GlnR regulon: identification of new GlnR targets and evidence for a central role of GlnR in nitrogen metabolism in actinomycetes. Mol Microbiol 67:861–880
Tiffert Y, Franz-Wachtel M, Fladerer C, Nordheim A, Reuther J, Wohlleben W, Mast Y (2011) Proteomic analysis of the GlnR-mediated response to nitrogen limitation in Streptomyces coelicolor M145. Appl Microbiol Biotechnol 89:1149–1159
Tseng HC, Chen CW (1991) A cloned ompR-like gene of Streptomyces lividans 66 suppresses defective melC1, a putative copper-transfer gene. Mol Microbiol 5:1187–1196
Urem M, van Rossum T, Bucca G, Moolenaar GF, Laing E, Swiatek-Polatynska MA, Willemse J, Tenconi E, Rigali S, Goosen N, Smith CP, van Wezel GP (2016) OsdR of Streptomyces coelicolor and the dormancy regulator DevR of Mycobacterium tuberculosis control overlap** regulons. Msystems 1:e0001416
Wang CX, Ge HX, Dong HJ, Zhu CG, Li YQ, Zheng J, Cen PL (2007) A novel pair of two-component signal transduction system ecrE1/ecrE2 regulating antibiotic biosynthesis in Streptomyces coelicolor. Biologia 62:511–516
Wang J, Zhao GP (2009) GlnR positively regulates nasA transcription in Streptomyces coelicolor. Biochem Biophys Res Commun 386:77–81
Wang WH, Shu D, Chen L, Jiang WH, Lu YH (2009) Cross-talk between an orphan response regulator and a noncognate histidine kinase in Streptomyces coelicolor. Fed Eur Microbiol Soc Microbiol Lett 294:150–156
Wang Y, Cen XF, Zhao GP, Wang J (2012) Characterization of a new GlnR binding box in the promoter of amtB in Streptomyces coelicolor inferred a PhoP/GlnR competitive binding mechanism for transcriptional regulation of amtB. J Bacteriol 194:5237–5244
Wang R, Mast Y, Wang J, Zhang WW, Zhao GP, Wohlleben W, Lu YH, Jiang WH (2013) Identification of two-component system AfsQ1/Q2 regulon and its cross-regulation with GlnR in Streptomyces coelicolor. Mol Microbiol 87:30–48
Wang GJ, Izawa M, Yang XG, Xu DB, Tanaka Y, Ochi K (2017) Identification of a novel lincomycin resistance mutation associated with activation of antibiotic production in Streptomyces coelicolor. Antimicrob Agents Chemother 61 A3(2):e0224716
Wei W, Wang WH, Cao ZW, Yu H, Wang XJ, Zhao J, Tan H, Xu H, Jiang WH, Li YX (2007) Comparative analysis of two-component signal transduction system in two streptomycete genomes. Acta Biochim Biophys Sin 39:317–325
West AH, Stock AM (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci 26:369–376
Yao LL, Liao CH, Huang G, Zhou Y, Rigali S, Zhang BC, Ye BC (2014) GlnR-mediated regulation of nitrogen metabolism in the actinomycete Saccharopolyspora erythraea. Appl Microbiol Biotechnol 98:7935–7948
Yeo KJ, Han YH, Eo Y, Cheong HK (2013a) Expression, purification, crystallization and preliminary X-ray analysis of the extracellular sensory domain of DraK histidine kinase from Streptomyces coelicolor. Acta Crystallogr Sect F Struct Biol Cryst Commun 69:909–911
Yeo KJ, Kim EH, Hwang E, Han YH, Eo Y, Kim HJ, Kwon O, Hong YS, Cheong C, Cheong HK (2013b) pH-dependent structural change of the extracellular sensor domain of the DraK histidine kinase from Streptomyces coelicolor. Biochem Biophys Res Commun 431:554–559
Yepes A, Rico S, Rodriguez-Garcia A, Santamaria RI, Diaz M (2011) Novel two-component systems implied in antibiotic production in Streptomyces coelicolor. PLoS ONE 6:e19980
Yu ZY, Zhu H, Dang FJ, Zhang WW, Qin ZJ, Yang S, Tan HR, Lu YH, Jiang WH (2012) Differential regulation of antibiotic biosynthesis by DraR-K, a novel two-component system in Streptomyces coelicolor. Mol Microbiol 85:535–556
Yu ZY, Zhu H, Zheng GS, Jiang WH, Lu YH (2014) A genome-wide transcriptomic analysis reveals diverse roles of the two-component system DraR-K in the physiological and morphological differentiation of Streptomyces coelicolor. Appl Microbiol Biotechnol 98:9351–9363
Zhang PP, Wu LL, Zhu YP, Liu M, Wang YM, Cao GX, Chen XL, Tao MF, Pang XH (2017) Deletion of MtrA inhibits cellular development of Streptomyces coelicolor and alters expression of developmental regulatory genes. Front Microbiol 8:2013
Zheng GS, Liu PP, He WY, Tao HN, Yang Z, Sun CW, Wang WF, Lu YH, Jiang WH (2021) Identification of the cognate response regulator of the orphan histidine kinase OhkA involved in both secondary metabolism and morphological differentiation in Streptomyces coelicolor. Appl Microbiol Biotechnol 105:5905–5914
Zhu Y, Zhang P, Zhang J, Xu W, Wang X, Wu L, Sheng D, Ma W, Cao G, Chen X-L, Lu Y, Zhang Y-Z, Pang X (2019) The developmental regulator MtrA binds GlnR boxes and represses nitrogen metabolism genes in Streptomyces coelicolor. Mol Microbiol 112:29–46
Zhu YP, Zhang PP, Zhang J, Wang J, Lu YH, Pang XH (2020) Impact on multiple antibiotic pathways reveals MtrA as a master regulator of antibiotic production in Streptomyces spp. and potentially in other Actinobacteria. Appl Environ Microbiol 86:e0120120
Zhu YP, Zhang PP, Lu T, Wang XY, Li AY, Lu YH, Tao MF, Pang XH (2021) Impact of MtrA on phosphate metabolism genes and the response to altered phosphate conditions in Streptomyces. Environ Microbiol 23:6907–6923
Zhu YP, Wang XY, Zhang J, Ni X, Zhang X, Tao MF, Pang XH (2022) The regulatory gene wblA is a target of the orphan response regulator OrrA in Streptomyces coelicolor. Environ Microbiol 24:3081–3096
Acknowledgements
This work was financially supported by the Doctoral Scientific Research Start-up Foundation from Henan University of Technology (No. 2019BS056), Key scientific research projects of universities in Henan Province (No. 23A180007).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
**, S., Hui, M., Lu, Y. et al. An overview on the two-component systems of Streptomyces coelicolor. World J Microbiol Biotechnol 39, 78 (2023). https://doi.org/10.1007/s11274-023-03522-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03522-6