Abstract
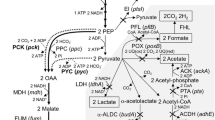
Escherichia coli pfl ldhA ptsG (AFP111) was studied for succinate production in defined medium using trace gases typically found in flue gas; i.e. oxygen (O2), nitrogen dioxide (NO2), sulfur dioxide (SO2) and carbon monoxide (CO). Following aerobic cell growth, cells were exposed to 50% CO2 and 3–10% O2, or 50–300 ppm NO2, SO2 or CO during the succinate production phase. Although 3% O2 did not significantly affect succinate formation, 10% O2 reduced the final succinate concentration from 33 to 17 g/L, specific productivity from 1.90 to 1.13 mmol/g h and yield from 1.15 to 0.81 mol/mol glucose. The effect of O2 correlated with the culture redox potential (ORP) with more reducing conditions favoring succinate production. The trace gases NO2 and SO2 also reduced the rate of succinate formation by as much as 50%, and led to a greater than twofold increase in pyruvate formation. Similar concentrations of CO showed no effect on succinate production rate or yield. Using synthetic flue gas AFP111 generated 12 g/L succinate with a specific productivity of 0.73 mmol/g h and a yield of 0.65 mol/mol.


Similar content being viewed by others
References
Battino R (ed) (1981) Solubility data series, vol 7, oxygen and ozone. Pergamon Press, Oxford
Browning DF, Beatty CM, Wolfe AJ, Cole JA, Busby SJ (2002) Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol Microbiol 43:687–701. doi:10.1046/j.1365-2958.2002.02776.x
Browning DF, Lee DJ, Wolfe AJ, Cole JA, Busby SJ (2006) The Escherichia coli K-12 NarL and NarP proteins insulate the nrf promoter from the effects of integration host factor. J Bacteriol 188:7449–7456. doi:10.1128/JB.00975-06
Cairns J, Denhardt DT (1968) Effect of cyanide and carbon monoxide on the replication of bacterial DNA in vivo. J Mol Biol 36:335–342. doi:10.1016/0022-2836(68)90159-9
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67:148–154. doi:10.1128/AEM.67.1.148-154.2001
Cole JA, Wimpenny JW (1968) Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta 162:39–48. doi:10.1016/0005-2728(68)90212-0
Donnelly MI, Millard CS, Clark DP, Chen MJ, Rathke JW (1998) A novel fermentation pathway in an Escherichia coli mutant producing succinic acid, acetic acid, and ethanol. Appl Biochem Biotechnol 70–72:187–198. doi:10.1007/BF02920135
Eiteman MA, Chastain MJ (1997) Optimization of the ion-exchange analysis of organic acids from fermentation. Anal Chim Acta 338:69–75. doi:10.1016/S0003-2670(96)00426-6
Garrett RH, Grisham CM (1999) Biochemistry. Saunders College Publishing, Fort Worth
Kaiser M, Sawers G (1994) Pyruvate formate-lyase is not essential for nitrate respiration by Escherichia coli. J Bacteriol 177(13):3647–3655
Kosaka H, Yamamoto K, Oda Y, Uozumi M (1986) Induction of SOS functions by nitrogen dioxide in Escherichia coli with different DNA-repair capacities. Mutat Res 162:1–5. doi:10.1016/0027-5107(86)90065-5
Kumari S, Tishel R, Eisenbach M, Wolfe AJ (1995) Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol 177:2878–2886
Lu S, Eiteman MA, Altman E (2009) Effect of CO2 on succinate production in dual-phase Escherichia coli fermentations. J Biotechnol 143:213–223
Mat-Jan F, Alam KY, Clark DP (1989) Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol 171:342–348
Matsumura H, **e Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y (2002) Crystal structures of C4 form maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases. Struct Camb 10:1721–1730
Matte A, Tari LW, Goldie H, Delbaere LTJ (1997) Structure and mechanism of phosphoenolpyruvate carboxykinase. J Biol Chem 272:8105–8108. doi:10.1074/jbc.272.13.8105
McKinlay J, Vieille C, Zeikus J (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Modak HV, Kelly DJ (1995) Acetyl-CoA-dependent pyruvate carboxylase from the photosynthetic bacterium Rhodobacter capsulatus: rapid and efficient purification using dye-ligand affinity chromatography. Microbiology 141:2619–2628
Mukai F, Hawryluk I, Shapiro R (1970) The mutagenic specificity of sodium bisulfite. Biochem Biophys Res Commun 39(5):983–988
Mukerji SK, Yang SF (1974) Phosphoenolpyruvate carboxylase from spinach leaf tissue: inhibition by sulfite ion. Plant Physiol 53:829–834
Orsini RA, Sarro TL, Wilson JA (1981) Source test and evaluation report: D. H. Mitchell unit No.11, Northern Indiana Public Service Co. Research Triangle Park, NC: U. S. Environmental Protection Agency, Industrial Environmental Research Laboratory; Cincinnati, OH: Center for Environmental Research Information
Osmond CB, Avadhani PN (1970) Inhibition of the beta-carboxylation pathway of CO2 fixation by bisulfite compounds. Plant Physiol 45:228–230
Ostrowski J, Barber MJ, Rueger DC, Miller BE, Siegel LM, Kredich NM (1989) Characterization of the flavoprotein moieties of NADPH-sulfite reductase from Salmonella typhimurium and Escherichia coli. Physicochemical and catalytic properties, amino acid sequence deduced from DNA sequence of cysJ, and comparison with NADPH-cytochrome P-450 reductase. J Biol Chem 264:15796–15808
Peguin S, Soucaille P (1996) Modulation of metabolism of Clostridium acetobutylicum grown in chemostat culture. Biotechnol Bioeng 51:342–348
Piantadosi CA (2002) Biological chemistry of carbon monoxide. Antioxid Redox Signal 4:259–270
Prohl C, Wackwitz B, Vlad D, Unden G (1998) Functional citric acid cycle in an arcA mutant of Escherichia coli during growth with nitrate under anoxic conditions. Arch Microbiol 170:1–7
Robakis N, Rossman T, Shapiro R, Szer W (1983) The effects of bisulfite on growth and macromolecular synthesis in Escherichia coli. Chem Biol Interact 43:289–298
Scinto LL, Maddalone RF, McNeil DK, Wilson JA (1981) Source test and evaluation report; Cane Run Unit No. 6, Louisville Gas and Electric Co. Research Triangle Park, NC: U.S. Environmental Protection Agency, Industrial Environmental Research Laboratory; Cincinnati, OH: Center for Environmental Research Information
Siegel LM, Davis PS, Kamin H (1974) Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem 249:1572–1586
Singhal RP (1971) Modification of Escherichia coli glutamate transfer ribonucleic acid with bisulfite. J Biol Chem 246(18):5848–5851
Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234
Vemuri GN, Eiteman MA, Altman E (2002a) Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68:1715–1727
Vemuri GN, Eiteman MA, Altman E (2002b) Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J Ind Microbiol Biotechnol 28:325–332
Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72(5):3653–3661
Weigel PH, Englund PT (1975) Inhibition of DNA replication in Escherichia coli by cyanide and carbon monoxide. J Biol Chem 250:8536–8542
Wimpenny JW, Cole JA (1967) The regulation of metabolism in facultative bacteria. III. The effect of nitrate. Biochim Biophys Acta 148:233–242
Acknowledgments
We thank US Department of Energy (DE-FG26-04NT42126) and Georgia Experiment Stations for financial support. We acknowledge S. A. Lee and R. Altman for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, S., Eiteman, M.A. & Altman, E. Effect of flue gas components on succinate production and CO2 fixation by metabolically engineered Escherichia coli . World J Microbiol Biotechnol 26, 429–435 (2010). https://doi.org/10.1007/s11274-009-0185-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0185-1