Abstract
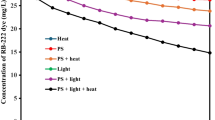
The degradation of Ester-105 by potassium persulfate activation in three ways (light, heat and microwave) was studied by simulating organic pollutants in wastewater. The removal effects of different factors including concentrations of persulfate and pollutant, pH of the reaction solution, light intensity/heat temperature/microwave power, typical inorganic cations and anions on the three systems were investigated, and the dynamic simulation was also carried out to compare and analyze the influence mechanism of each factor. As the result, under the conditions of 20 mg/L Ester-105 and 1.5 mmol/L potassium persulfate, the optimum reaction conditions were 100 W mercury lamp, 60 ℃, and 260 W of microwave power for light, heat, and microwave system respectively. The strong alkali inhibited the photoactivation and reduced the reaction rate about 30%; both of the strong acid and strong alkali in the thermal activation system promoted the reaction, and their reaction rate constants are almost twice of the unregrulated pH (6.0); pH had little effect on the microwave activation. The addition of Fe2+ showed a very strong promoting effect on three reaction systems, especially in the photoactivation that higher than 90% of Ester-105 was removed in 1 min. SiO32− was beneficial to the degradation of Ester-105 in the photoactivation system while other ainions almost played an inhibitory role in all systems. The main active free radicals involved in the reaction were SO4−● and ●OH through the free radical trap** experiment. The TOC removal efficiency and the activated rate of persulfate were also in agreement with degradation effect of Ester-105. Varieties of intermediates such as carbonyl sulfide and 3-ethoxyacrylonitrile were detected by GC–MS.
Graphical Abstract










Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Chen, C., Wang, C., Zhou, H., Chen, R., He, P., & Zhao, Y. (2014). GC-MS Analysis of Secondary Pollutant from Several Beneficiation Reagents. Chemical Reagents, 36(01), 51–53. https://doi.org/10.13822/j.cnki.hxsj.2014.01.025
Chen, H., Chen, H., Wang, Q., Liu, T., Xu, L. (2017a). A case study of treatment and renovation of wastewater from production of strong alkaline and high sulfur ion mineral processing agents. Technology of Water Treatment, 43(10). https://doi.org/10.16796/j.cnki.1000-3770.2017.10.033.
Chen, H., Zhang, Z., Feng, M., Liu, W., Wang, W., Yang, Q., & Hu, Y. (2017b). Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chemical Engineering Journal, 313, 498–507. https://doi.org/10.1016/j.cej.2016.12.075
Chen, T., Ma, J., Zhang, Q., **e, Z., Zeng, Y., Li, R., Liu, H., Liu, Y., Lv, W., Liu, G. (2019b). Degradation of propranolol by UV-activated persulfate oxidation: Reaction kinetics, mechanisms, reactive sites, transformation pathways and Gaussian calculation. Science of the Total Environment, 690. https://doi.org/10.1016/j.scitotenv.2019.07.034.
Chen, X., Yang, B., Oleszczuk, P., Gao, Y., Yuan, X., Ling, W., Waigi, M.-G. (2019a). Vanadium oxide activates persulfate for degradation of polycyclic aromatic hydrocarbons in aqueous system. Chemical Engineering Journal, 364. https://doi.org/10.1016/j.cej.2019.01.117.
Chen, G., Yang, Y., Lan, L., **aoguang, D., Rui, L., Xukai, L., Beibei, Y., Ning, L., & Shaobin, W. (2020). Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. Journal of Hazardous Materials, 408, 124461. https://doi.org/10.1016/j.jhazmat.2020.124461
Dominguez, C.M., Rodriguez, V., Montero, E., Romero, A., Santos.(2020) A. Abatement of dichloromethane using persulfate activated by alkali: A kinetic study. Separation and Purification Technology, 241(C). https://doi.org/10.1016/j.seppur.2020.116679.
Du, X., Bai, X., Xu, L., Yang, L., **, P. (2020). Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chemical Engineering Journal, 384. https://doi.org/10.1016/j.cej.2019.123245.
Emine, C.-G., Yesim, D., Senem, Y.-G., Farshid, G., Gamze, V. (2022). Effective removal of furfural by ultraviolet activated persulfate, peroxide, and percarbonate oxidation: Focus on influencing factors, kinetics, and water matrix effect. Journal of Photochemistry & Photobiology, A: Chemistry, 433. https://doi.org/10.1016/J.JPHOTOCHEM.2022.114139.
Ghorbanian, Z., Asgari, G., Samadi, M.-T., Seid-mohammadi, A. (2019). Removal of 2,4 dichlorophenol using microwave assisted nanoscale zero-valent copper activated persulfate from aqueous solutions: Mineralization, kinetics, and degradation pathways. Journal of Molecular Liquids, 296. https://doi.org/10.1016/j.molliq.2019.111873.
Guo, Y., Gao, N., Guan, X., Zhu, S., Lu, X., An, N. (2017). Degradation of diclofenac sodium in water by ultraviolet activated persulfate. Journal of Harbin Institute of Technology, 49(8). https://doi.org/10.11918/j.issn.0367-6234.20160412 .
Hanxuan, Z., Shuwen, S., Anhong, C., Qian, S., Lei, W., Shijun, Z., Xueyan, L., **g, D. (2022). Degradation of tetracycline by UV/Fe3+/persulfate process: Kinetics, mechanism, DBPs yield, toxicity evaluation and bacterial community analysis. Chemosphere, 307. https://doi.org/10.1016/J.CHEMOSPHERE.2022.136072.
He, L., Jiahao, L., **, F. (2023). Efficient degradation and detoxification of antibiotic Fosfomycin by UV irradiation in the presence of persulfate. Science of the Total Environment, 905. https://doi.org/10.1016/j.scitotenv.2023.167249 .
Kim, J. S., Kumar, N., Jung, U., Park, J., Naushad, M. (2023). Enhanced photocatalytic activity of cubic ZnSn(OH)6 by in-situ partial phase transformation via rapid thermal annealing. Chemosphere, 331. https://doi.org/10.1016/j.chemosphere.2023.138780 .
Lee, M.-Y., Wang, W.-L., Xu, Z.-B., Ye, B., Wu, Q.-Y., Hu, H.-Y. (2019). The application of UV/PS oxidation for removal of a quaternary ammonium compound of dodecyl trimethyl ammonium chloride (DTAC): The kinetics and mechanism. Science of the Total Environment, 655. https://doi.org/10.1016/j.scitotenv.2018.11.256.
Lei, Y.-J., Tian, Y., Sobhani, Z., Naidu, R., Fang, C. (2020). Synergistic degradation of PFAS in water and soil by dual-frequency ultrasonic activated persulfate. Chemical Engineering Journal, 388(C). https://doi.org/10.1016/j.cej.2020.124215.
Li, H. (2015). Methods and engineering applications of mineral processing wastewater treatment and reuse. Hydrometallurgy of China, 34(6). https://doi.org/10.13355/j.cnki.sfyj.2015.06.001.
Lin, H., Ai, J., Li, R., Deng, L., Tan, W., Ye, Z., Wu, X., & Zhang, H. (2020). Treatment of organosilicon wastewater by UV-based advanced oxidation processes: Performance comparison and fluorescence parallel factor analysis. Chemical Engineering Journal, 380, 122536. https://doi.org/10.1016/j.cej.2019.122536
Lin, Y., Xu, H., Su, B., Su, Y., Yang, H., Bai, C., Rui, C. (2021). Ultraviolet light activates persulfate to degrade triclosan in water. Journal of Environmental Science, 40(4). https://doi.org/10.13623/j.cnki.hkdk.2021.04013 .
Liu, X., Liu, Y., Lu, S., Wang, Z., Wang, Y., Zhang, G., Guo, X., Guo, W., Zhang, T., **, B. (2020). Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chemical Engineering Journal, 385. https://doi.org/10.1016/j.cej.2019.123987.
Olga S. A., Alexandra A. I., Angeliki P., Kleopatra M., Ioannis K., Dionissios, M., Zacharias, F. (2022). Dexamethasone degradation in aqueous medium by a thermally activated persulfate system: Kinetics and transformation products. Journal of Water Process Engineering, 49. https://doi.org/10.1016/J.JWPE.2022.103134 .
Shirish, S., P, R.-M., K, L.-V., Kirill, F., Grzegorz, B. (2022). Thermally activated persulfate-based Advanced Oxidation Processes — recent progress and challenges in mineralization of persistent organic chemicals: a review. Current Opinion in Chemical Engineering, 37. https://doi.org/10.1016/J.COCHE.2022.100839.
Shuyu, L., Chunyun, G., Jiaxin, Z., Chaoyi, L., Xun, R., Gangsen, Y., Hanyu, L., **g, W., Jie, M. (2022). Degradation of 1,2,3-trichloropropane by unactivated persulfate and the implications for groundwater remediation. The Science of the total environment, 865. https://doi.org/10.1016/J.SCITOTENV.2022.161201.
Tiansheng C., Ma, J., Zhang, Q., **e, Z., Zeng, Y., Li, R., Liu, H., Liu, Y., Lv, W., Liu, G. (2019). Degradation of propranolol by UV-activated persulfate oxidation: Reaction kinetics, mechanisms, reactive sites, transformation pathways and Gaussian calculation. Science of the Total Environment, 690. https://doi.org/10.1016/j.scitotenv.2019.07.034 .
Wan, Z., Pan, J., Wang, C., Yu, W. (2022). Study on the treatment of benzohydroxamic acid by photoassisted persulfate oxidation. Industrial Water Treatment, 42(07). https://doi.org/10.19965/j.cnki.iwt.2021-0961.
Wancheng, P., Jun, Y., Šolević, K.-T., Ying, C., Bang, L., Hao, L., Miaomiao, L., Junjie, Z. (2023). Degradation of three typical hydroxamic acids collectors via UVA-B activated H2O2 and persulfate: Kinetics, transformation pathway, DFT calculation and toxicity evaluation. Chemical Engineering Journal, 451. https://doi.org/10.1016/J.CEJ.2022.138639.
Wang, Y., Guo, S., Gu, S., & Zhang, A. (2020). Comparison study on microwave irradiation-activated persulfate and hydrogen peroxide systems in the treatment of dinitrodiazophenol industrial wastewater. Chemosphere, 242, 125139. https://doi.org/10.1016/j.chemosphere.2019.125139
Wang, Q., Siyu, L., **n, W., Zhuoyu, L., Yali, Z., & Chunmao, C. (2022a). Efficient Degradation of 4-Acetamidoantipyrin Using a Thermally Activated Persulfate System. Sustainability, 14(21), 14300. https://doi.org/10.3390/SU142114300
Wang, L., Yu, Y., Liu, G., Hu, B., Lu, J.(2022b). Degradation of Tetrabromobisphenol S by Thermo-activated Persulfate Oxidation: Reaction Kinetics, Transformation Mechanisms, and Brominated By-products. Environmental Technology, 11(10). https://doi.org/10.1080/09593330.2022.2135027 .
**an-zhong, B., Yuan-yuan, F., Ji-wei, X., Lu, Y.,Chong-hui, Z. (2022). Effective recovery of chalcopyrite at low temperatures using modified Ester collector. Transactions of Nonferrous Metals Society of China, 32(1). https://doi.org/10.1016/S1003-6326(22)65795-9 .
**aoyan, D., **n, S., **ng, C., Da, D., Chang, X., Hong, C. (2021a). Degradation and mechanism of hexafluoropropylene oxide dimer acid by thermally activated persulfate in aqueous solutions. Chemosphere, 286. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131720 .
**aoyan, M., Liangjie, T., **g, D., Zhanghua, L., Xueyan, L., Peng, W., & Qingsong, L. (2021b). Removal of saccharin by UV/persulfate process: Degradation kinetics, mechanism and DBPs formation. Journal of Photochemistry and Photobiology a: Chemistry. https://doi.org/10.1016/J.JPHOTOCHEM.2021.113482
**, L., Jia, Z. (2022). Kinetics of zero-valent iron-activated persulfate for methylparaben degradation and the promotion of Cl−. Journal of Environmental Management, 321. https://doi.org/10.1016/J.JENVMAN.2022.115973.
Yang, Y., Banerjee, G., Brudvig, G. W., Kim, J. H., Pignatello, J. J. (2018). Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Journal of Environmental Science & Technology, 52(10). https://doi.org/10.1021/acs.est.8b00735 .
Yegane, B.M., Ali, E., Hasan, P., Rezaei, K.-R., Ehsan, A., Mitra, G., Ali, A. (2019). Degradation of dimethyl phthalate using persulfate activated by UV and ferrous ions: optimizing operational parameters mechanism and pathway. Journal of environmental health science & engineering, 17(2). https://doi.org/10.1007/s40201-019-00384-9.
Yu K., Wang Z., Chen L. (2022). Comparative study on the degradation of dimethyl phthalate by different advanced oxidation techniques. Journal of Applied Chemical Industry, 51(12). https://doi.org/10.16581/j.cnki.issn1671-3206.20221109.003.
Yunmei, W., Shuang, C., Tingting, R., Lianying, C., Yuanyuan, L., Junmin, G., Yunyi, L. (2021). Effectiveness and mechanism of cyanide remediation from contaminated soils using thermally activated persulfate. Chemosphere, 292. https://doi.org/10.1016/J.CHEMOSPHERE.2021.13346.
Ziyu, Z., **n, H., **ngle, G., Chunhong, J., Baotong, L., Ercheng, Z., Junxue, W. (2022). Efficient degradation of imidacloprid in soil by thermally activated persulfate process: Performance, kinetics, and mechanisms. Ecotoxicology and Environmental Safety, 241. https://doi.org/10.1016/J.ECOENV.2022.113815.
Acknowledgements
This research was financially supported by Natural Science Foundation of China (52360025), Jiangxi Provincial Natural Science Foundation (20232BAB203040), Qingjiang Excellent Young Talents Support Project of Jiangxi University of Science and Technology (JXUSTQJYX2016003), National College Student Innovation and Entrepreneurship Training Program (202010407004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yiting Zeng, Hui Qiu. The first draft of the manuscript was written by Yiting Zeng and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Y., Qiu, H., Zeng, J. et al. Degradation of Beneficiation Reagent Ester-105 by Light, Heat, and Microwave Activated Persulfate. Water Air Soil Pollut 235, 98 (2024). https://doi.org/10.1007/s11270-024-06906-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06906-y