Abstract
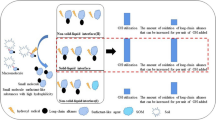
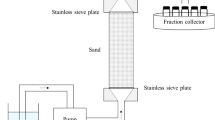
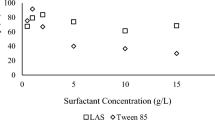
This work evaluated surfactant-enhanced in situ chemical oxidation (S-ISCO) in a hydrocarbon-contaminated soil. Surfactants and efficacy of oxidant activation as well as the treatability of contaminated soil were assessed. The surfactant VeruSOL-3 with a critical micelle concentration (CMC) of 5.5 g/L was selected. Based on the results, activated oxidations by sodium persulphate and hydrogen peroxide were able to effectively destroy target organic compounds in emulsion and soil. The destruction of total petroleum hydrocarbon (TPH) in emulsion was completed in 14 days and polycyclic aromatic hydrocarbons (PAHs) in excess of 96 %. Green nanoiron was much more active than other activators in emulsion. The data also indicates that oxidation using activators was much less pronounced in soil matrices. However, it is expected that given sufficient dose and treatment time, a higher destruction rate in the contaminated soil can be achieved. The study showed that the remediation of target organic contaminants (TPH, PAH) in soil by S-ISCO using activated sodium persulphate is feasible.






Similar content being viewed by others
References
Banerjee, D., Apollo, F. M., Ryabov, A. D., & Collins, T. J. (2009). The impact of surfactants on Fe(III)-TAML-catalyzed oxidations by peroxides: accelerations, decelerations, and loss of activity. Chemistry, 15(39), 10199–10209.
Baruwati, B., & Varma, R. S. (2009). High value products from waste: grape pomace extract—a three-in-one package for the synthesis of metal nanoparticles. Chemsuschem, 2(11), 1041–1044.
Crimi, M. L., & Taylor, J. (2007). Experimental evaluation of catalyzed hydrogen peroxide and sodium persulfate for destruction of BTEX contaminants. Soil & Sediment Contamination, 16(1), 29–45.
Dahmani, M., Huang, K., & Hoag, G. (2006). Sodium persulfate oxidation for the remediation of chlorinated solvents (USEPA Superfund Innovative Technology Evaluation Program). Water, Air, & Soil Pollution: Focus, 6(1), 127–141.
Dugan, P. J., Siegrist, R. L., & Crimi, M. L. (2010). Coupling surfactants/cosolvents with oxidants for enhanced DNAPL removal: a review. Remediation Journal, 20(3), 27–49.
Fallis, I. A. P. C., Griffiths, T., Cosgrove, C. A., Dreiss, N., Govan, R. K., Heenan, et al. (2009). Locus-specific microemulsion catalysts for sulfur mustard (HD) chemical warfare agent decontamination. Journal of the American Chemical Society, 131(28), 9746–9755.
Ferrarese, E., Andreottola, G., & Oprea, I. A. (2008). Remediation of PAH-contaminated sediments by chemical oxidation. Journal of Hazardous Materials, 152(1), 128–139.
Fountain, J. C., Starr, R. C., Middleton, T., Beikirch, M., Taylor, C., & Hodge, D. (1996). A controlled field test of surfactant-enhanced aquifer remediation. Ground Water, 34(5), 910–916.
Häger, M., Olsson, U., & Holmberg, K. (2004). A nucleophilic substitution reaction performed in different types of self-assembly structures. Langmuir, 20(15), 6107–6115.
Hoag, G. E. & Collins, J. B. (2011). Soil remediation method and composition. US Patent No. 7976241B2.
Hoag, G. E., Collins, J. B., Holcomb, J. L., Hoag, J. R., Nadagoudab, M. N., & Varma, R. S. (2009). Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. Journal of Materials Chemistry, 19, 8671–8677.
Hoag, G. E., Collins, J. B., Varma, R. S., & Nadagoudab, M. N. (2011). Green synthesis of nanometals using plant extracts and use thereof. US Patent No. 8057682B2.
Huang, K.-C., Zhao, Z., Hoag, G. E., Dahmani, A., & Block, P. A. (2005). Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere, 61(4), 551–560.
Huh, C. (1979). Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. Journal of Colloid and Interface Science, 71(2), 408–426.
Kanan, K., Yousef, H., & Kayali, I. (2012). Nanostructured microemulsion phase behavior using AOT or extended surfactant combined with a cationic hydrotrope. Journal of Surface Engineered Materials and Advanced Technology, 2, 53–60.
Li, F., Vipulanandan, C., & Mohanty, K. K. (2003). Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 223(1–3), 103–112.
Liang, C., & Bruell, C. J. (2008). Thermally activated persulfate oxidation of trichloroethylene: experimental investigation of reaction orders. Industrial and Engineering Chemistry Research, 47(9), 2912–2918.
Liang, C., Huang, C.-F., & Chen, Y.-J. (2008a). Potential for activated persulfate degradation of BTEX contamination. Water Research, 42(15), 4091–4100.
Liang, C., Huang, C.-F., Mohanty, N., & Kurakalva, R. M. (2008b). A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere, 73(9), 1540–1543.
Liang, C. J., Bruell, C. J., Marley, M. C., & Sperry, K. L. (2003). Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1,1,1-trichloroethane (TCA) in aqueous systems and soil slurries. Soil & Sediment Contamination, 12(2), 207.
Liu, S. H., Zhang, D. L., Yan, W., Puerto, M., Hirasaki, G. J., & Miller, C. A. (2008). Favorable attributes of alkaline-surfactant-polymer flooding. SPE Journal, 13(1), 5–16.
Nadagouda, M. N., Castle, A. B., Murdock, R. C., Hussain, S. M., & Varma, R. S. (2010). In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea-polyphenols. Green Chemistry, 12(1), 114–122.
Nadagouda, M. N., Hoag, G., Collins, J. B., & Varma, R. S. (2009). Green synthesis of Au nanostructures at room temperature using biodegradable plant surfactants. Crystal Growth & Design, 9(11), 4979–4983.
Njagi, E., Huang, C. H., Stafford, L., Genuino, H., Galindo, H. M., Collins, J. B., et al. (2011). Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Cleanup 2011—4th International Contaminated Site Remediation Conference (Adelaide, Australia), pp. 71–72
von Sonntag, C. (2008). Advanced oxidation processes: mechanistic aspects. Water Science & Technology—WST, 58(5), 1015–1021.
Tsitonaki, A., Petri, B., Crimi, M., MosbÆK, H., Siegrist, R. L., & Bjerg, P. L. (2010). In situ chemical oxidation of contaminated soil and groundwater using persulfate: a review. Critical Reviews in Environmental Science & Technology, 40(1), 55–91.
Zhang, W.-x. (2003). Nanoscale iron particles for environmental remediation: an overview. Journal of Nanoparticle Research, 5, 323–332.
Zhou, T., Li, Y., Ji, J., Wong, F.-S., & Lu, X. (2008). Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: kinetic, pathway and effect factors. Separation and Purification Technology, 62(3), 551–558.
Acknowledgments
Dr. Wei Hong Wang gratefully acknowledges CRC CARE (Australia) for funding this project. The author would also like to thank VeruTEK Company (USA) for the opportunity to co-work on this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: R Naidu, Euan Smith, MH Wong, Megharaj Mallavarapu, Nanthi Bolan, Albert Juhasz, and Enzo Lombi
This article is part of the Topical Collection on Remediation of Site Contamination
Rights and permissions
About this article
Cite this article
Wang, W.H., Hoag, G.E., Collins, J.B. et al. Evaluation of Surfactant-Enhanced In Situ Chemical Oxidation (S-ISCO) in Contaminated Soil. Water Air Soil Pollut 224, 1713 (2013). https://doi.org/10.1007/s11270-013-1713-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1713-z