Abstract
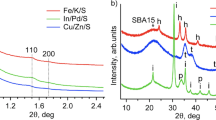
The present work has investigated influence of pore structure of silica coated on copper-zinc oxide-based catalyst for hydrogenation of carbon dioxide into methanol. For controlling the pore structure, micelles of a cationic surfactant, cethyltrimethylammonium bromide (CTAB), was used without and with a swelling agent, 1-dodecylamine, during coating silica on the commercial copper–zinc oxide-based catalyst. The pore structure depended on the amount of the surfactant and the swelling agent, and the specific surface area and the ratio of mesopores of the silica-coated catalyst significantly increased with adding 1-dodecylamine with the surfactant. The silica-coated catalyst prepared both with CTAB and 1-dodecylamine showed the significantly rapid evaporation of physisorbed water on the catalysts, and the property can reflect the catalyst’s high activity and stability for hydrogenation of carbon dioxide into methanol.






Similar content being viewed by others
References
Tlili A, Frogneux X, Blondiaux E, Cantat T (2014) Creating Added Value with a Waste: Methylation of Amines with CO2 and H2. Angew Chem Int Ed 53:2543–2545. https://doi.org/10.1002/anie.201310337
Goeppert A, Czaun M, Jones JP, Prakash GKS, Olah GA (2014) Recycling of carbon dioxide to methanol and derived products—closing the loop. Chem Soc Rev 43:7995–8048. https://doi.org/10.1039/C4CS00122B
Artz J, Müller TE, Thenert K, Kleinekorte J, Meys R, Sternberg A, Bardow A, Leitner W (2018) Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem Rev 118:434–504. https://doi.org/10.1021/acs.chemrev.7b00435
Li Y, Cui X, Dong K, Junge K, Beller M (2017) Utilization of CO2 as a C1 building block for catalytic methylation reactions. ACS Catal 7:1077–1086. https://doi.org/10.1021/acscatal.6b02715
Olah GA, Goeppert A, Prakash GKS (2009) Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J Org Chem 74:487–498. https://doi.org/10.1021/jo801260f
Bartholomew CH, Farrauto RJ (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, Hoboken, pp 83–391
Wu J, Luo S, Toyir J, Saito M, Takeuchi M, Watanabe T (1998) Optimization of preparation conditions and improvement of stability of Cu/ZnO-based multicomponent catalysts for methanol synthesis from CO2 and H2. Catal Today 45:215–220. https://doi.org/10.1016/S0920-5861(98)00218-1
Wu J, Saito M, Mabuse H (2000) Activity and stability of Cu/ZnO/Al2O3 catalyst promoted with B2O3 for methanol synthesis. Catal Lett 68:55–58. https://doi.org/10.1023/A:1019010831562
Zha F, Ding J, Chang Y, Ding J, Wang J, Ma J (2012) Cu-Zn-Al oxide cores packed by metal-doped amorphous silica-alumina membrane for catalyzing the hydrogenation of carbon dioxide to dimethyl ether. Ind Eng Chem Res 51:345–352. https://doi.org/10.1021/ie202090f
Umegaki T, Kojima Y, Omata K (2015) Effect of silica coating on performance of copper-zinc oxide based catalyst for methanol synthesis. Adv Mater Lett 6:1026–1030. https://doi.org/10.5185/amlett.2015.5558
Umegaki T, Kojima Y, Omata K (2015) Effect of oxide coating on performance of copper-zinc oxide-based catalyst for methanol synthesis via hydrogenation of carbon dioxide. Materials 8:7738–7744. https://doi.org/10.3390/ma8115414
Lee JF, Lee N, Kim T, Kim J, Hyeon T (2011) Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res 44:893–902. https://doi.org/10.1021/ar2000259
Fang X, Liu Z, Hsieh MF, Chen M, Liu P, Chen C, Zheng N (2012) Hollow mesoporous aluminosilica spheres with perpendicular pore channels as catalytic nanoreactors. ACS Nano 6:4434–4444. https://doi.org/10.1021/nn3011703
Li X, John VT, He G, He J, Spinu L (2012) Magnetic TiO2–SiO2 hybrid hollow spheres with TiO2 nanofibers on the surface and their formation mechanism. J Mater Chem 22:17476–17484. https://doi.org/10.1039/C2JM31967E
Awual MR, Hasan MM (2015) Fine-tuning mesoporous adsorbent for simultaneous ultra-trace palladium(II) detection, separation and recovery. J Ind Eng Chem 21:507–515. https://doi.org/10.1016/j.jiec.2014.03.013
Zou H, Wang R, Shi Z, Dai J, Zhang Z, Qiu S (2016) One-dimensional periodic mesoporous organosilica helical nanotubes with amphiphilic properties for the removal of contaminants from water. J Mater Chem A 4:4145–4154. https://doi.org/10.1039/C6TA00708B
Umegaki T, Ogawa R, Toyama N, Ohki S, Tansho M, Shimizu T, Kojima Y (2017) The influence of the pore structure of hollow silica–alumina composite spheres on their activity for hydrolytic dehydrogenation of ammonia borane. Inorg Chem Front 4:1568–1574. https://doi.org/10.1039/C7QI00338B
Umegaki T, Ogawa R, Ohki S, Tansho M, Shimizu T, Kojima Y (2019) Control of pore size in shell of hollow silica–alumina composite spheres for hydrolytic dehydrogenation of ammonia borane. J Porous Mater 26:611–617. https://doi.org/10.1007/s10934-018-0665-5
Cao L, Man T, Kruk M (2009) Synthesis of ultra-large-pore SBA-15 silica with two-dimensional hexagonal structure using triisopropylbenzene as micelle expander. Chem Mater 21:1144–1153. https://doi.org/10.1021/cm8012733
Ceratti DR, Faustini M, Sinturel C, Vayer M, Dahirel V, Jardat M, Grosso D (2015) Critical effect of pore characteristics on capillary infiltration in mesoporous films. Nanoscale 7:5371–5382. https://doi.org/10.1039/c4nr03021d
Chen H, Hu T, Zhang X, Huo K, Chu PK, He J (2010) One-step synthesis of monodisperse and hierarchically mesostructured silica particles with a thin shell. Langmuir 26:13556–13563. https://doi.org/10.1021/la101778z
Wang X, Zhang Y, Luo W, Elzatahry AA, Cheng X, Alghamdi A, Abdullah AM, Deng Y, Zhao D (2016) Synthesis of ordered mesoporous silica with tunable morphologies and pore sizes via a nonpolar solvent-assisted stöber method. Chem Mater 28:2356–2362. https://doi.org/10.1021/acs.chemmater.6b00499
Ernawati L, Balgis R, Ogi T, Okuyama K (2017) Tunable synthesis of mesoporous silica particles with unique radially oriented pore structures from tetramethyl orthosilicate via oil-water emulsion process. Langmuir 33:783–790. https://doi.org/10.1021/acs.langmuir.6b04023
Umegaki T, Watanabe K, Ogawa H, Kojima Y (2020) Influence of swelling agents on pore size distributions of porous silica-alumina hollow sphere particles in acid-promoted hydrolytic generation of hydrogen from ammonia borane. Int J Hydrogen Energy 45:19531–19538. https://doi.org/10.1016/j.ijhydene.2020.05.042
Acknowledgements
This work was supported by an ALCA (Advanced Low Carbon Technology Research and Development) project of Japan Science Technology Agency (JST) for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Umegaki, T., Kojima, Y. & Omata, K. Influence of Pore Structure of Silica Coated on Copper-Zinc Oxide-Based Catalyst for Carbon Dioxide into Methanol. Top Catal 64, 576–581 (2021). https://doi.org/10.1007/s11244-021-01430-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01430-3