Abstract
Background
The study and development of fluorouracil metal complexes are important in the development of new synthetic methods and materials with applications in pharmaceuticals, agrochemicals, and materials science.
Mothodology
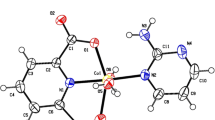
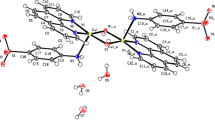
A new Cd(II) compound, (H-5FC) [(H-5FC) Cd Cl ] (1), (where H-5FC is HFlucytosine), was successfully synthesized and crystallized by slow evaporation at room temperature. The compound was characterized by single-crystal X-ray diffraction technique and UV–Visible spectroscopy.
Results
The structure shows that the compound constitutes of an independent protonated (H-5FC)+ cation and two protonated flucytosine molecules that coordinate to the Cd(II) ion via an oxygen atom to form a trinuclear [(H-5FC)2Cd3Cl10]2− anionic moieties. The independent protonated (H-5FC)+ bridges the [(H-5FC)2Cd3Cl10]2− anions via N/C–H···Cl/O hydrogen bonds. Supramolecular structure analysis of (1) with the aid of Hirshfeld calculations showed the importance of the H···Cl, O···H, C···Cl, and F···Cl interactions. Their percentages were calculated to be 42.2, 10.3, 6.6, and 8.7%, respectively. The band gap energy of the compound, deduced from the Tauc plot of the absorption spectrum, indicated a wide energy gap of 3.65 eV.





Similar content being viewed by others
References
Hulme AT, Tocher DA (2006) Cryst Growth Des 6:481–487
Tilborg A, Norberg B, Wouters J (2014) Eur J Med Chem 74:411–426
Mohana M, Thomas MP, McMillen CD, Butcher RJ (2023) Acta Crystallog Sect C 79:61–67
Surampudi AVSD, Ramakrishna S, Pallavi A, Balasubramanian S (2023) CrystEngComm 25:1220–1231
Nechipadappu SK, and Balasubramanian S (2023) Acta Crystallographica section b: structural science, Crystal Eng Mater 79
Firmino PP, Pedro HDO, da Silva CC, de Araujo-Neto JH and Ellena J (2023) J Molecul Struct 136075
Mohapatra B, Verma S (2017) Chem Commun 53:4748–4758
Lippert B, Müller J (2016) Inorg Chim Acta 100:1–2
Sander SA, Morrow JR (2016) Inorg Chim Acta 452:90–97
Bartova S, Alberti E, Sigel RK, Donghi D (2016) Inorg Chim Acta 452:104–110
Capllonch MC, Garcı́a-Raso A, Terrón A, Apella MC, Espinosa E, Molins E (2001) J Inorg Biochem 85:173–178
Muthiah PT, Robert JJ, Raj SB, Bocelli G, Ollá R (2001) Acta Crystallogr Sect E: Struct Rep Online 57:m558–m560
Stanley N, Muthiah PT, Luger P, Weber M, Geib S (2005) Inorg Chem Commun 8:1056–1059
Waalkes MP, Poirier LA (1984) Toxicol Appl Pharmacol 75:539–546
Khandar AA, Azar ZM, Eskandani M, Hubschle CB, van Smaalen S, Shaabani B, Omidi Y (2019) Polyhedron 171:237–248
Luo H-Y, Li J-Y, Li Y, Zhang L, Li J-Y, Jia D-Z, Xu G-C (2016) RSC Adv 6:114997–115009
Fedorov B, Fadeev M, Utenyshev A, Shilov G, Konovalova N, Tatyanenko L, Sashenkova T, Blokhina S, Berseneva E (2011) Russian Chem Bull 60:1959–1962
Montazerozohori M, Zahedi S, Nasr-Esfahani M, Naghiha A (2014) J Ind Eng Chem 20:2463–2470
You ZL, Zhu HL (2006) Z Anorg Allg Chem 632:140–146
Netalkar PP, Netalkar SP, Revankar VK (2015) Polyhedron 100:215–222
Azam M, Khan AA, Al-Resayes SI, Islam MS, Saxena AK, Dwivedi S, Musarrat J, Trzesowska-Kruszynska A, Kruszynski R (2015) Spectrochim Acta Part A Mol Biomol Spectrosc 142:286–291
Grunberg E, Titsworth E, Bennett M (1964) Chemotherapeutic activity of 5-fluorocytosine 566–568
Vermes A, Guchelaar H-J, Dankert J (2000) J Antimicrob Chemotherapy 46:171–179
Benson J, Nahata M (1988) Clin Pharm 7:424–438
Nyati M, Symon Z, Kievit E, Dornfeld K, Rynkiewicz S, Ross B, Rehemtulla A, Lawrence T (2002) Gene Ther 9:844–849
Delma FZ, Al-Hatmi AM, Brüggemann RJ, Melchers WJ, de Hoog S, Verweij PE, Buil JB (2021) J Fungi 7:909
Portalone G, Colapietro M (2006) Acta Crystallogr Sect E Struct Rep Online 62:o1049–o1051
Tutughamiarso M, Bolte M, Egert E (2009) Acta Crystallogr C 65:o574–o578
Da Silva CC, de Oliveira R, Tenorio JC, Honorato SB, Ayala AP, Ellena J (2013) Cryst Growth Des 13:4315–4322
Prabakaran P, Murugesan S, Muthiah PT, Bocelli G, Righi L (2001) Acta Crystallogr Sect E Struct Rep Online 57:o933–o936
Portalone G, Colapietro M (2007) J Chem Crystallogr 37:141–145
Perumalla SR, Pedireddi VR, Sun CC (2013) Cryst Growth Des 13:429–432
Perumalla SR, Pedireddi VR, Sun CC (2013) Mol Pharm 10:2462–2466
Portalone G (2011) Chem Cent J 5:1–8
Tutughamiarso M, Wagner G, Egert E (2012) Acta Crystallogr B 68:431–443
Tutughamiarso M, Egert E (2012) Acta Crystallogr B 68:444–452
Bruker BAI (2016) APEX3 Crystallography Software Suite, Madison, vol 2016. WI, USA
Bruker A, Saint A (2008) Acta Crystallogr Sect A Fundam Crystallogr 64:112
Sheldrick G (2015) Acta Crystallogr A 71:3–8
Sheldrick G (2015) Acta Crystallogr C 71:3–8
Sadabs BAI (2017) Madison. Wisconsin, USA
Farrugia L (1997) J Appl Crystallogr 30:565
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) J Appl Crystallogr 53:226–235
Qin L-L, Ye H-Y, Wang D-Y (2014) Inorg Chem Commun 46:47–50
Sun X-F, Wang Z, Liao W-Q, Li P-F, Gao J, Huang Y-Y, Chen H-P, Ye H-Y, Zhang Y (2017) RSC Adv 7:52024–52029
Karthikeyan A, Zeller M, Thomas Muthiah P (2018) Acta Crystallogr Sect C Struct Chem 74:789–796
Silverstein RM, Bassler GC (1962) J Chem Educ 39:546
Palafox MA, Rastogi VK (2015) Asian Chem Lett 19:01–2533
Gunasekaran S, Seshadri S, Muthu S (2006) Indian J Pure Appl Phys 44:581–586
Sharma V, Sharma S, Sharma V (1995) Asian J Chem 7:855
Seshadri S, Gunasekaran S, Muthu S, Kumaresan S, Arunbalaji R (2007) J Raman Spectro Int J Orig Work Asp Raman Spectro Includ Higher Order Process Brillouin Rayleigh Scatter 38:1523–1531
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19–32
Spackman MA, McKinnon JJ (2002) CrystEngComm 4:378–392
Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Jayatilaka D, Spackman MA (2021) J Appl Crystallogr 54:1006–1011
Cavalieri LF, Bendich A (1950) J Am Chem Soc 72:2587–2594
Makuła P, Pacia M, Macyk W (2018) ACS Publications, pp. 6814–6817
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023114. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Author information
Authors and Affiliations
Contributions
H.F. and D.C.DO. wrote the main manuscript text, M.F analyzed the structure by SCXRD and refined the structure, N.S. A and N.P. R prepared figures and data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11243_2023_562_MOESM1_ESM.docx
Supplementary file1 (DOCX 1859 KB): CCDC-2249554 contains the supplementary crystallographic data for this paper. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Additional figures and tables (Figures 1S to 5S and scheme 1; Tables 1S) pertaining to the structure and spectra are given in electronic supplementary information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferjani, H., Almotlaq, N.S., Fettouhi, M. et al. A novel Cd(II) compound of flucytosine: synthesis, structure, and optical properties. Transit Met Chem 49, 67–74 (2024). https://doi.org/10.1007/s11243-023-00562-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-023-00562-7