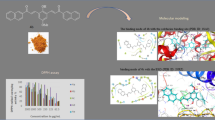
Abstract
The reaction of 1-phenyl-1,3-butanedione, also known as benzoylacetone (bzac), with adequate copper salts (sulfate/acetate) at a molar ratio of 2:1 in methanol led to two mononuclear complexes, trans-[Cu(bzac)2] (I) and cis-[Cu(bzac)2(CH3OH)] (II). Both complexes crystallize in the monoclinic P21/c symmetry. The copper is four- and five-coordinate, exhibiting a square planar geometry and a distorted square-based pyramid in I and II, respectively. Their crystal structures form discrete supramolecular packing. Indeed, Hirshfeld surface analysis (HSA) with 2D fingerprint plots revealed short-range intermolecular contacts involving O—H···Ο hydrogen bonds and C—H···π interactions in both complexes, in addition to π···π interactions in I. The complexes were characterized by IR and UV‒Vis spectroscopic methods. Moreover, a thorough examination of I and II was conducted, focusing on their structural attributes, electronic characteristics, and both linear and nonlinear optical (NLO) responses through density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. These quantum calculations were executed utilizing uωwb97xd/6-311G**/SDD. The results revealed that the β0 value for II was approximately 23 times greater than that of urea. On the other hand, the static and dynamic second hyperpolarizabilities (γ(0; 0,0,0), γ(− 2ω; ω,0,0), and γ(− 2ω; ω,ω,0)) of I are approximately 33% higher than those of II. From this, we infer that the complexes under investigation have potential as outstanding materials for second- and third-order NLO applications. The interactions of I and II with tubulin (PDB ID: 4O2B) were evaluated by molecular docking studies. The results showed that both complexes can bind to many sites on the target and may inhibit its polymerization process. Furthermore, the antioxidant activity of both complexes was also determined and fully discussed.



















Similar content being viewed by others
Data availability
The crystallographic details are provided in the CIFs available from the Cambridge Crystallographic Data Centre at https://www.ccdc.cam.ac.uk/structures/ with CCDC numbers: 2196130 and 2196139 for compounds I and II, respectively. The protein’s crystal structure was obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank at https://www.rcsb.org with the following Protein Data Bank (PDB) ID: 4O2B.
References
Allen G, Dwek RA (1966) An nmr study of keto–enol tautomerism in β-diketones. J Chem Soc B, p 161-163
Yamabe S, Tsuchida N, Miyajima K (2004) Reaction paths of keto− enol tautomerization of β-diketones. J Phys Chem A 108(14):2750–2757
Sloop JC, Bumgardner CL, Washington G, Loehle WD, Sankar SS, Lewis AB (2006) Keto–enol and enol–enol tautomerism in trifluoromethyl-β-diketones. J Fluorine Chem 127(6):780–786
Da Silva MR, Ferrao MLC (1988) Energetics of metal-oxygen bonds in metal complexes of β-diketones. Pure Appl Chem 60(8):1225–1234
Heinemann F (1997) Synthesis and structures of platina-β-diketonato complexes of platina-β-diketones; organometallic analogues of platinum blue complexes. Chem Commun 9:843–844
Skopenko VV, Amirkhanov VM, Sliva TY, Vasilchenko IS, Anpilova E, Garnovskii AD (2004) Various types of metal complexes based on chelating β-diketones and their structural analogues. Russ Chem Rev 73(8):737–752
Priya NP, Arunachalam SV, Sathya N, Chinnusamy V, Jayabalakrishnan C (2009) Catalytic and antimicrobial studies of binuclear ruthenium (III) complexes containing bis-β-diketones. Transition Met Chem 34(4):437–445
Holm R, Cotton F (1958) Spectral investigations of metal complexes of β-diketones. I. Nuclear magnetic resonance and ultraviolet spectra of acetylacetonates1. J Am Chem Soc 80(21):5658–5663
Okafor EC (1981) The metal complexes of heterocyclic β-diketones and their derivatives, part VIII synthesis, structure, proton NMR and infrared spectral studies of the complexes of Al (III), Fe (III), Co (III), Rh (III), In (III), and Zr (IV) with l-phenyl-3-methyl-4-trifluoroacetyl-pyrazolone-5 (HPMTFP). Zeitschrift für Naturforschung B 36(2):213–217
Heller ST, Natarajan SR (2006) 1, 3-Diketones from acid chlorides and ketones: a rapid and general one-pot synthesis of pyrazoles. Org Lett 8(13):2675–2678
Polshettiwar V, Varma RS (2008) Greener and rapid access to bio-active heterocycles: room temperature synthesis of pyrazoles and diazepines in aqueous medium. Tetrahedron Lett 49(2):397–400
** W, Yu H, Yu Z (2011) Regioselective synthesis of multisubstituted pyrazoles via cyclocondensation of β-thioalkyl-α, β-unsaturated ketones with hydrazines. Tetrahedron Lett 52(44):5884–5887
Joule JA, Mills K, Smith GF (2020) Heterocyclic chemistry. CRC Press
Stille JK, Unglaube J, Freeburger M (1968) Reactions of cyclic 1, 3-diketones with hydrazine. Mechanism of cinnolino [5, 4, 3-cde] cinnoline formation. Unusual oxidation as a result of steric crowding. J Am Chem Soc 90(25):7076–7083
Prasse C, von Gunten U, Sedlak DL (2020) Chlorination of phenols revisited: unexpected formation of α, β-unsaturated C4-dicarbonyl ring cleavage products. Environ Sci Technol 54(2):826–834
Wypych G (2015) 2-PVC properties. PVC formulary, pp 5–44
Houska J, Salhi E, Walpen N, von Gunten U (2021) Oxidant-reactive carbonous moieties in dissolved organic matter: selective quantification by oxidative titration using chlorine dioxide and ozone. Water Res 207:117790
Lim S, Shi JL, von Gunten U, McCurry DL (2022) Ozonation of organic compounds in water and wastewater: a critical review. Water Research, p 118053
Zhang G, Wu B, Zhang S (2017) Effects of acetylacetone on the photoconversion of pharmaceuticals in natural and pure waters. Environ Pollut 225:691–699
**e M, Zhang C, Zheng H, Zhang G, Zhang S (2022) Peroxyl radicals from diketones enhanced the indirect photochemical transformation of carbamazepine: kinetics, mechanisms, and products. Water Res 217:118424
Vigato PA, Peruzzo V, Tamburini S (2009) The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord Chem Rev 253(7–8):1099–1201
Kljun J, Turel I (2017) β-Diketones as scaffolds for anticancer drug design–from organic building blocks to natural products and metallodrug components. Eur J Inorg Chem 2017(12):1655–1666
Sharma N, Kumari M, Kumar V, Chaudhry S (2010) Synthesis, structural characterisation and antibacterial activity of bis (1-phenyl-1, 3-butanedionato) non-oxovanadium (IV) hydroxamates. J Enzyme Inhib Med Chem 25(5):708–714
Kaushal R, Thakur S (2016) Nehra K (2016) ct-DNA Binding and Antibacterial Activity of Octahedral Titanium (IV) Heteroleptic (Benzoylacetone and Hydroxamic Acids) Complexes. Int J Med Chem 1:2361214
Ekennia AC, Onwudiwe DC, Olasunkanmi LO, Osowole AA (2015) Ebenso EE (2015) Synthesis, DFT calculation, and antimicrobial studies of novel Zn (II), Co (II), Cu (II), and Mn (II) heteroleptic complexes containing benzoylacetone and dithiocarbamate. Bioinorg Chem Appl 1:789063
Pais GC, Zhang X, Marchand C, Neamati N, Cowansage K, Svarovskaia ES, Pathak VK, Tang Y, Nicklaus M, Pommier Y (2002) Structure activity of 3-aryl-1, 3-diketo-containing compounds as HIV-1 integrase inhibitors. J Med Chem 45(15):3184–3194
Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M (1998) Effect of the beta-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7, 12-dimethylbenz [a] anthracene. Carcinogenesis 19(6):1039–1043
Pettinari R, Marchetti F, Di Nicola C, Pettinari C (2018) Half-sandwich metal complexes with β-diketone-like ligands and their anticancer activity. Eur J Inorg Chem 2018(31):3521–3536
Eshaghi Malekshah R, Salehi M, Kubicki M, Khaleghian A (2019) Biological studies and computational modeling of two new copper complexes derived from β-diketones and their nano-complexes. J Coord Chem 72(10):1697–1714
Wilson JJ, Lippard SJ (2012) In vitro anticancer activity of cis-diammineplatinum (II) complexes with β-diketonate leaving group ligands. J Med Chem 55(11):5326–5336
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334(2):115–124
Semmingsen D, Larsson LO, Nyberg B, Huhtikangas A, Pearson W, Meisalo V (1972) The Crystal Structure of Benzoylacetone. Acta Chemi Scand 26:143–154
Jones RD (1976) The crystal and molecular structure of the enol form of 1-phenyl-1, 3-butanedione (benzoylacetone) by neutron diffraction. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem 32(7):2133–2136
Schiøtt B, Iversen BB, Hellerup Madsen GK, Bruice TC (1998) Characterization of the short strong hydrogen bond in benzoylacetone by ab initio calculations and accurate diffraction experiments Implications for the electronic nature of low-barrier hydrogen bonds in enzymatic reactions. J Am Chem Soc 120(46):12117–12124
Madsen GK (1999) Structure and bonding in Cis-enol systems. In: AIP Conference Proceedings. American Institute of Physics 479:107–111
Tayyari SF, Emampour J, Vakili M, Nekoei AR, Eshghi H, Salemi S, Hassanpour M (2006) Vibrational assignment and structure of benzoylacetone: a density functional theoretical study. J Mol Struct 794(1–3):204–214
Matsuzawa H, Nakagaki T, Iwahashi M (2007) Intramolecular hydrogen bonding (proton transfer) of 1-phenyl-1, 3-butanedione. J Oleo Sci 56(12):653–658
Durlak P, Latajka Z (2014) Car-Parrinello and path integral molecular dynamics study of the intramolecular hydrogen bonds in the crystals of benzoylacetone and dideuterobenzoylacetone. Phys Chem Chem Phys 16(42):23026–23037
Kidd M, Sager RS, Watson WH (1967) Properties of some copper (II) and zinc (II) N-oxide and B-diketone complexes. Inorg Chem 6(5):946–951
Belford RC, Fenton D, Truter MR (1972) Reactions of 1, 4-diazabicyclo [2, 2, 2] octane with bis-(1, 1, 1, 5, 5, 5-hexafluoropentane-2, 4-dionato) copper (II) and the crystal structure of the 1: 1 complex. J Chem Soc, Dalton Trans 20:2208–2213
Siedle A, Newmark R, Pignolet L (1982) Structure and stereochemical nonrigidty in the triarylphosphinepalladium bis (hexafluoroacetylacetonate), a new isomeric class of palladium bis (hexafluoroacetylacetonate) complexes. J Am Chem Soc 104(24):6584–6590
Soldatov DV, Ripmeester JA (2000) Inclusion in microporous β-Bis (1, 1, 1-trifluoro-5, 5-dimethyl-5-methoxyacetylacetonato) copper (II), an organic zeolite mimic. Chem Mater 12(7):1827–1839
Maverick AW, Fronczek FR, Maverick EF, Billodeaux DR, Cygan ZT, Isovitsch RA (2002) Structures of anhydrous and hydrated copper (II) hexafluoroacetylacetonate. Inorg Chem 41(24):6488–6492
Sahbari J, Olmstead M (1983) Structure of sodium acetylacetonate monohydrate, Na [C5H7O2]. H2O. Acta Crystallogr Sect C Cryst Struct Commun 39(8):1037–1038
Vicente J, Chicote MAT (1999) The ‘acac method’for the synthesis of coordination and organometallic compounds: synthesis of gold complexes. Coordination Chem Rev 193:1143–1161
Rzepa HS, Cass ME (2007) In search of the Bailar and Rây− Dutt twist mechanisms that racemize chiral trischelates: a computational study of ScIII, TiIV, CoIII, ZnII, GaIII, and GeIV complexes of a ligand analogue of acetylacetonate. Inorg Chem 46(19):8024–8031
Suttil J, Kucharyson J, Escalante-Garcia I, Cabrera P, James B, Savinell R, Sanford M, Thompson L (2015) Metal acetylacetonate complexes for high energy density non-aqueous redox flow batteries. Journal of Materials Chemistry A 3(15):7929–7938
**ong R-G, Zuo J-L, Yu Z, You X-Z, Chen W (1999) Eu5 (μ4-OH)(μ3-OH) 4 (μ-DBM) 4 (DBM) 6 (DBM= dibenzoylmethide): a novel Eu5 square-pyramid polynuclear complex with a rare μ4-OH bridging mode. Inorg Chem Commun 2(10):490–494
Zeng X-R, **ong R-G, You X-Z, Cheung K-K (2000) Triboluminescent spectrum and crystal structure of a europate complex with the most intensely triboluminescent emission at ambient temperature. Inorg Chem Commun 3(7):341–344
**ong R-G, You X-Z (2002) Synthesis and characterization of the firstly observed two brilliantly triboluminescent lanthanide complexes: 2-hydroxyethylammonium and pyrrolidinium tetrakis (dibenzoylmethide) europate (III). Crystal structure of one brilliantly triboluminescent acentric complex: dimethylbenzylammonium tetrakis (dibenzoylmethide) europate. Inorg Chem Commun 5(9):677–681
Jung YS, Lee JH, Song K, Kang S-J (1998) The structural study of the lithium beta-diketonate complex. Bull Korean Chem Soc 19(4):484–486
Hon P-K, Pfluger CE, Belford RL (1967) Bis (1-phenyl-1, 3-butanedionato) palladium (II). Crystal and molecular structure of the trans form. Inorg Chem 6(4):730–735
Knyazeva A, Shugam E, Shkol’nikova, L (1970) Crystal chemical data regarding intracomplex compounds of beta diketones. J Struct Chem 11(5):875–876
Hon P-K, Pfluger C, Belford RL (1966) The molecular and crystal structure of bis (1-phenyl-l, 3-butanedionato) copper. Inorg Chem 5(4):516–521
Dey SK, Bag B, Zhou Z, Chan AS, Mitra S (2004) Synthesis, characterization and crystal structure of a monomeric and a macrocyclic copper (II) complex with a large cavity using benzylacetylacetone ligand. Inorg Chim Acta 357(7):1991–1996
Krishnegowda HM, Karthik CS, Marichannegowda MH, Kumara K, Kudigana PJ, Lingappa M, Mallu P, Neratur LK (2019) Synthesis and structural studies of 1-phenyl-1, 3-butanedione copper (II) complexes as an excellent antimicrobial agent against methicillin-resistant Staphylococcus aureus. Inorg Chim Acta 484:227–236
Cox E, Shorter A, Wardlaw W (1937) Stereochemistry of bivalent tin and lead. Nature 139(3506):71–72
Serpone N, Fay RC (1967) Stereochemistry and lability of dihalobis (beta-diketonato) titanium (IV) complexes. II Benzoylacetonates and dibenzoylmethanates Inorganic Chemistry 6(10):1835–1843
Hambley TW, Hawkins CJ, Kabanos TA (1987) Synthetic, structural, and physical studies of tris (2, 4-pentanedionato) vanadium (IV) hexachloroantimonate (V) and tris (1-phenyl-1, 3-butanedionato) vanadium (IV) hexachloroantimonate (V). Inorg Chem 26(22):3740–3745
Liu R, Conradie J (2015) Tris (β-diketonato) chromium (III) complexes: effect of the β-diketonate ligand on the redox properties. Electrochim Acta 185:288–296
Freitag R, Conradie J (2015) Electrochemical and computational chemistry study of Mn (β-diketonato) 3 complexes. Electrochim Acta 158:418–426
Conradie MM, Van Rooyen PH, Conradie J (2016) X-ray and electronic structure of tris (benzoylacetonato-κ2O, O’) iron (III). J Mol Struct 1123:199–205
Liu R, Van Rooyen PH, Conradie J (2016) Geometrical isomers of tris (β-diketonato) metal (III) complexes for M= Cr or Co: synthesis, X-ray structures and DFT study. Inorg Chim Acta 447:59–65
Saxena U, Rai A, Mathur V, Mehrotra R, Radford D (1970) Reactions of zirconium isopropoxide with β-diketones and β-keto-esters. J Chem Soc A, p 904-907
Zehra S, Tabassum S, Arjmand F (2021) Biochemical pathways of copper complexes: progress over the past 5 years. Drug Discovery Today 26(4):1086–1096
Marzano C, Pellei M, Tisato F, Santini C (2009) Copper complexes as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry (formerly current medicinal chemistry-anti-cancer agents) 9(2):185–211
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Advances in copper complexes as anticancer agents. Chem Rev 114(1):815–862
Dustin P (2012) Microtubules. Springer Science & Business Media
Parker AL, Kavallaris M, McCarroll JA (2014) Microtubules and their role in cellular stress in cancer. Front Oncol 4:153
Caporizzo MA, Chen CY, Prosser BL (2019) Cardiac microtubules in health and heart disease. Exp Biol Med 244(15):1255–1272
Waites C, Qu X, Bartolini F (2021) The synaptic life of microtubules. Curr Opin Neurobiol 69:113–123
MacRae TH (1992) Towards an understanding of microtubule function and cell organization: an overview. Biochem Cell Biol 70(10–11):835–841
Borisy G, Heald R, Howard J, Janke C, Musacchio A, Nogales E (2016) Microtubules: 50 years on from the discovery of tubulin. Nat Rev Mol Cell Biol 17(5):322–328
Sharp DJ, Rogers GC, Scholey JM (2000) Microtubule motors in mitosis. Nature 407(6800):41–47
Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH (2002) Microtubule structure at 8 Å resolution. Structure 10(10):1317–1328
Conde C, Cáceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10(5):319–332
McLoughlin EC, O’Boyle NM (2020) Correction: McLoughlin, EC; O’Boyle (2020) NM Colchicine-binding site inhibitors from chemistry to clinic: a review. Pharmaceuticals 13(4):72
D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E (1994) 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci 91(9):3964–3968
Ben-Chetrit E (1998) Colchicine: 1998 update. Semin Arthritis Rheum 28:48–59
Prinz H (2002) Recent advances in the field of tubulin polymerization inhibitors. Expert Rev Anticancer Ther 2(6):695–708
Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC (2003) Microtubule-interacting drugs for cancer treatment. Trends Pharmacol Sci 24(7):361–365
Bruker A (2017) SAINT, Version 8.40B, Bruker analytical X-ray systems. Inc., Madison, Wisconsin, USA
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Crystallogr 48(1):3–10
Sheldrick GM (2015) SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Adv 71(1):3–8
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallographica Section C: Structural Chemistry 71(1):3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42(2):339–341
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4(66):378–392
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816
Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Jayatilaka D, Spackman MA (2021) CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J Appl Crystallogr 54(3):1006–1011
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Kargar H, Fallah-Mehrjardi M, Ashfaq M, Munawar KS, Tahir MN, Behjatmanesh-Ardakani R, Rudbari HA, Ardakani AA, Sedighi-Khavidak S (2021) Zn (II) complexes containing O, N, N, O-donor Schiff base ligands: synthesis, crystal structures, spectral investigations, biological activities, theoretical calculations and substitution effect on structures. J Coord Chem 74(16):2720–2740
Spackman MA, Byrom PG (1997) A novel definition of a molecule in a crystal. Chem Phys Lett 267(3–4):215–220
Ramalingam A, Kansız S, Dege N, Sambandam S (2021) Synthesis, crystal structure, DFT calculations and Hirshfeld surface analysis of 3-chloro-2, 6-bis (4-chlorophenyl)-3-methylpiperidin-4-one. J Chem Crystallogr 51:273–287
Ashfaq M, Bogdanov G, Ali A, Tahir MN, Abdullah S (2021) Pyrimethamine-based novel co-crystal salt: synthesis, single-crystal investigation, Hirshfeld surface analysis and DFT inspection of the 2, 4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium 2, 4-dichlorobenzoate (1: 1)(DECB). J Mol Struct 1235:130215
Taia A, Ibrahimi BE, Benhiba F, Ashfaq M, Tahir MN, Essaber M, Aatif A, Hökelek T, Mague JT, Sebbar NK, Essassi EM (2021) Syntheses, single crystal X-ray structure, Hirshfeld surface analyses, DFT computations and Monte Carlo simulations of New Eugenol derivatives bearing 1, 2, 3-triazole moiety. J Mol Struct 1234:130189
Chavda BR, Socha BN, Pandya SB, Chaudhary KP, Padariya TJ, Alalawy MD, Patel MK, Dubey RP, Patel UH (2021) Coordination behavior of dinuclear silver complex of sulfamethoxazole with solvent molecule having static rotational disorder: spectroscopic characterization, crystal structure, Hirshfeld surface and antimicrobial activity. J Mol Struct 1228:129777
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10(44):6615–6620
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128(8):084106
Hannachi D, Khelfaoui N, Zaidi M, Yahiaoui D, Lakehal S, Morell C, Chermette H (2023) The effect of resonance-assisted hydrogen bond on the second-order nonlinear optical properties of pyridine hydrazone photoswitches: a quantum chemistry investigation. New J Chem 47(39):18359–18373
Kamli D, Hannachi D, Samsar D, Chermette H (2023) Bis-TTF-Ge derivatives: promising linear and nonlinear optical properties, a theoretical investigation. New J Chem 47(3):1234–1246
Irshad S, Ullah F, Khan S, Ludwig R, Mahmood T, Ayub K (2021) First row transition metals decorated boron phosphide nanoclusters as nonlinear optical materials with high thermodynamic stability and enhanced electronic properties; a detailed quantum chemical study. Opt Laser Technol 134:106570
Iqbal J, Ayub K (2016) Theoretical study of the non linear optical properties of alkali metal (Li, Na, K) doped aluminum nitride nanocages. RSC Adv 6(96):94228–94235
Sarwar S, Yaqoob J, Khan MU, Hussain R, Zulfiqar S, Anwar A et al (2022) Deciphering the role of alkali metals (Li, Na, K) do** for triggering nonlinear optical (NLO) properties of t-graphene quantum dots: toward the development of giant NLO response materials. ACS Omega 7(28):24396–24414
Abegao LM, Fonseca RD, Santos FA, Rodrigues JJ, Kamada K, Mendonça CR et al (2019) First molecular electronic hyperpolarizability of series of π-conjugated oxazole dyes in solution: an experimental and theoretical study. RSC Adv 9(45):26476–26482
He HM, Yang H, Li Y, Li ZR (2022) Front Chem 10:1–10
Mravec B, Budzak S, Medved M, Pasteka LF, Slavov C, Sassmannshausen T et al (2021) Design of high-performance pyridine/quinoline hydrazone photoswitches. J Org Chem 86(17):11633–11646
Rice JE, Amos RD, Colwell SM, Handy NC, Sanz J (1990) Frequency dependent hyperpolarizabilities with application to formaldehyde and methyl fluoride. J Chem Phys 93(12):8828–8839
Kodikara MS, Stranger R, Humphrey MG (2018) Computational studies of the nonlinear optical properties of organometallic complexes. Coord Chem Rev 375:389–409
Kleinman DA (1962) Nonlinear dielectric polarization in optical media. Phys Rev 126(6):1977
Kurtz HA, Stewart JJ, Dieter KM (1990) Calculation of the nonlinear optical properties of molecules. J Comput Chem 11(1):82–87
Shelton DP, Rice JE (1994) Measurements and calculations of the hyperpolarizabilities of atoms and small molecules in the gas phase. Chem Rev 94(1):3–29
Bersohn R, Pao YH, Frisch H (1966) Double-quantum light scattering by molecules. J Chem Phys 45(9):3184–3198
Plaquet A, Guillaume M, Champagne B, Castet F, Ducasse L, Pozzo JL, Rodriguez V (2008) In silico optimization of merocyanine-spiropyran compounds as second-order nonlinear optical molecular switches. Phys Chem Chem Phys 10(41):6223–6232
Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998) A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76(2):270–276
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1):235–242
Prota AE, Danel F, Bachmann F, Bargsten K, Buey RM, Pohlmann J, Reinelt S, Lane H, Steinmetz MO (2014) The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol 426(8):1848–1860
Biovia, Dassault Systèmes B (2021) Discovery studio visualizer, v21. 1.0. 20298. Dassault Systèmes, San Diego CA USA
Hathaway B, Billing D (1970) The electronic properties and stereochemistry of mono-nuclear complexes of the copper (II) ion. Coord Chem Rev 5(2):143–207
Crutchley RJ, Hynes R, Gabe EJ (1990) Five-and four-coordinate copper (II) complexes of 2, 2’-bipyridine and phenylcyanamide anion ligands: crystal structures, cyclic voltammetry, and electronic absorption spectroscopy. Inorg Chem 29(24):4921–4928
Mateyise NGS, Ghosh S, Gryzenhout M, Chiyindiko E, Conradie MM, Langner EH, Conradie J (2021) Synthesis, characterization, DFT and biological activity of oligothiophene β-diketone and Cu-complexes. Polyhedron 205:115290
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC (1984) Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J Chem Soc, Dalton Trans 7:1349–1356
Liang YY, Li B, Xu X, Long Gu F, Zhu C (2019) A density functional theory study on nonlinear optical properties of double cage excess electron compounds: theoretically design M [Cu (Ag)@(NH3) n](M= Be, Mg and Ca; n= 1–3). J Comput Chem 40(9):971–979
Baggi N, Garoni E, Colombo A, Dragonetti C, Righetto S, Roberto D (2018) … & Fantacci, S, Design of cyclometallated 5-π-delocalized donor-1, 3-di (2-pyridyl) benzene platinum (II) complexes with second-order nonlinear optical properties. Polyhedron 140:74–77
Andraud C, Fortrie R, Barsu C, Stéphan O, Chermette H, Baldeck PL (2008) Excitonically coupled oligomers and dendrimers for two-photon absorption. Photoresponsive Polymers II:149–203
Khan ZR, Shkir M, Ganesh V, AlFaify S, Yahia IS, Zahran HY (2018) Linear and nonlinear optics of CBD grown nanocrystalline F doped CdS thin films for optoelectronic applications: an effect of thickness. J Electron Mater 47:5386–5395
Iliopoulos K, Krupka O, Gindre D, Sallé M (2010) Reversible two-photon optical data storage in coumarin-based copolymers. J Am Chem Soc 132(41):14343–14345
Homocianu M, Airinei A, Hamciuc C, Ipate AM (2019) Nonlinear optical properties (NLO) and metal ions sensing responses of a polymer containing 1, 3, 4-oxadiazole and bisphenol A units. J Mol Liq 281:141–149
Bencheikh K (2003) Spin–orbit coupling in the spin-current-density-functional theory. J Phys A: Math Gen 36(48):11929
Yahiaoui AA, Ghichi N, Hannachi D, Djedouani A, Meskaldji S, Merazig H, Harakat D (2022) Synthesis, XRD/HSA-interactions, biological activity, optical and nonlinear optical responses studies of new pyran derivative. J Mol Struct 1263:133161
Boucherabine D, Merzougui M, Hannachi D, Melchiorre M, Pinto G, Ouari K (2023) Oxovanadium and copper complexes of new unsymmetrically tetradentate ligands: X-ray structure, theoretical and NLO properties, catalytic oxidation and bromoperoxidase activities. J Mol Struct 1291:136053
Lamiri W, Djaafer-Moussa R, Merabet L, Setifi F, Kaboub L, Hannachi D (2023) Synthesis, XRD/HSA-interactions, QTAIM analysis, optical and nonlinear optical responses studies of Cu (II) complex with the Schiff base ligand derived from 5-bromosalicylaldehyde. ChemistrySelect 8(43):e202302953
Bree C, Demircan A, Steinmeyer G (2010) Method for computing the nonlinear refractive index via Keldysh theory. IEEE J Quantum Electron 46(4):433–437
Tarazkar M, Romanov D, Levis R (2014) Higher-order nonlinearity of refractive index: the case of argon. J Chem Phys 140(21):214316–214316
Dey SK, Bag B, Abdul Malik K, El Fallah MS, Ribas J, Mitra S (2003) A quasi-tetrahedral Cu4 cluster and a helical-chain copper (II) complex with single syn− anti carboxylate bridges: crystal structure and magnetic properties. Inorg Chem 42(13):4029–4035
Oda S, Kozuka H, Uchiyama H (2015) Thermoplastic softening behavior of organically modified polyoxotitanates: Effects of the amount of water and benzoylacetone for hydrolyzing alkoxides. J Appl Polym Sci 41(132)
Ashfaq M, Tahir MN, Muhammad S, Munawar KS, Ali A, Bogdanov G, Alarfaji SS (2021) Single-crystal investigation, Hirshfeld surface analysis, and DFT study of third-order NLO properties of unsymmetrical acyl thiourea derivatives. ACS Omega 6(46):31211–31225
Direm A, Abdelbaky MS, Sayın K, Cornia A, Abosede O, Garcia-Granda S (2018) Sev and pcu topological nets in one-pot newly synthesized mixed-ligand imidazole-containing Cu (II) coordination frameworks: crystal structure, intermolecular interactions, theoretical calculations, magnetic behavior and biological activity. Inorg Chim Acta 478:59–70
Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M (2004) Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428(6979):198–202
Nogales E, Wolf SG, Downing KH (1998) Erratum: Structure of the αβ tubulin dimer by electron crystallography. Nature 393(6681):191–191
Zheng YB, Gong JH, Liu XJ, Wu SY, Li Y, Xu XD et al (2016) A novel nitrobenzoate microtubule inhibitor that overcomes multidrug resistance exhibits antitumor activity. Sci Rep 6(1):31472
Acknowledgements
M.G. thanks Centro di Servizi di Cristallografia Strutturale (CRIST), University of Florence (Italy). The authors are very thankful to the University of Lyon, University of Claude Bernard Lyon 1, CNRS UMR 5280, Institute of Analytical Sciences, 69622 Villeurbanne Cedex, France, for offering the computing facilities.
Funding
The authors acknowledge the funding from the Ministry of Higher Education and Scientific Research (MESRS) and Abbes Laghrour University of Khenchela (Algeria) under project number: B00L01UN400120210002 (PRFU).
Author information
Authors and Affiliations
Contributions
Abdenour Guerraoui: writing—original draft, methodology, investigation, conceptualization, and software. Meriem Goudjil: writing—original draft, methodology, software, visualization, validation, and writing—review and editing. Amel Djedouani: visualization, validation, resources, and supervision. Amani Direm: software and validation. Abdelhalim Boussaa: software and validation. Douniazed Hannachi: writing—original draft, software, and validation. Elvira Fantechi: methodology and validation. Giampiero Ruani: visualization and validation. Abdecharif Boumaza: visualization, validation, resources, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guerraoui, A., Goudjil, M., Djedouani, A. et al. Cu(II)-bis(benzoylacetonate) complexes as potential inhibitors for tubulin polymerization: synthesis, crystal structure, spectral characterization, HSA, DFT, molecular docking studies, and antioxidant activity. Struct Chem (2024). https://doi.org/10.1007/s11224-024-02354-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-024-02354-w