Abstract
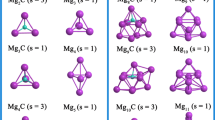
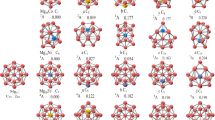
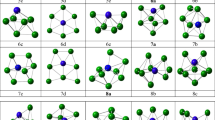
The interaction of molecular iodine on Mgn (n = 2–18) clusters has been investigated using first-principles calculations. Structural, adsorption energy and electronic properties of these systems are reported. After structure optimization, the iodine molecule undergoes dissociative adsorption, where the I–I covalent bond of molecular iodine is broken and the dissociative iodine atoms adsorb on the surfaces of the magnesium clusters. The adsorption energy ranging from − 4.335 to − 5.740 eV indicates the chemisorption of I on Mgn clusters. In the same way, for n > 4, Mg-I compounds have bond lengths of 2.694 to 2.937 Å forming ionic bonds and the values of charge transfer in MgnI2 reach − 0.829 to − 0.977 e. The projected density of states (PDOS) of Mg7I2, which has the highest absolute adsorption energy, and Mg16I2, which has the highest amount of charge transfer, demonstrate the strong hybridization between the Mg 3s and the I 5p orbitals. Overall, the change in electronic structure suggests that Mgn clusters might serve as promising adsorbents for the removal of gaseous radioactive iodine.








Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Knapp V, Pevec D (2018) Promises and limitations of nuclear fission energy in combating climate change. Energy Policy 120:94–99
Liu L, Guo H, Dai L, Liu M, **ao Y, Cong T, Gu H (2023) The role of nuclear energy in the carbon neutrality goal. Prog Nuclear Energy 162:104772
Pham TCT, Docao S, Hwang IC, Song MK, Choi DY, Moon D, Oleynikov P, Yoon KB (2016) Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ Sci 9:1050–1062
Sava DF, Rodriguez MA, Chapman KW, Chupas PJ, Greathouse JA, Crozier PS, Nenoff TM (2011) Capture of volatile iodine, a gaseous fission product, by zeolitic imidazolate framework-8. J Am Chem Soc 133:12398–12401
Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden JL (2016) Materials and processes for the effective capture and immobilization of radioiodine: A review. J Nucl Mater 470:307–326
Pei C, Ben T, Xu S, Qiu S (2014) Ultrahigh iodine adsorption in porous organic frameworks. J Mater Chem A 2:7179–7187
Garino TJ, Nenoff TM, Krumhansl JL, Rademacher DX (2011) Low-temperature sintering Bi-Si-Zn-Oxide glasses for use in either glass composite materials or core/shell 129I Waste Forms. J Am Ceram Soc 94:2412–2419
Shi M, Jiang G, Cheng X, Zhou X, Du J (2024) The capture performance of An-MOF for fission gases: Insight from DFT and AIMD calculations. Microporous Mesoporous Mater 363:112838
Tian Z, Chee T-S, Zhu L, Duan T, Zhang X, Lei L, **ao C (2021) Comprehensive comparison of bismuth and silver functionalized nickel foam composites in capturing radioactive gaseous iodine. J Hazard Mater 417:125978
Tristant D, Puech P, Gerber IC (2015) Theoretical study of graphene do** mechanism by iodine molecules. J Phys Chem C 119:12071–12078
Rudenko AN, Keil FJ, Katsnelson MI, Lichtenstein AI (2010) Adsorption of diatomic halogen molecules on graphene: A van der Waals density functional study. Phys Rev B 82:035427
Chibani S, Medlej I, Lebègue S, Ángyán JG, Cantrel L, Badawi M (2017) Performance of CuII-, PbII-, and HgII-Exchanged Mordenite in the Adsorption of I2, ICH3, H2O, CO, ClCH3, and Cl2: A Density Functional Study. ChemPhysChem 18:1642–1652
Zhang Z, Zhao Q, Huang M, Ouyang X (2019) Adsorption of hazardous gases in nuclear islands on monolayer MoS2 sheet. Adsorption 25:159–171
Cheng M, Zhang Z, Wang S, Bi K, Hu K-Q, Dai Z, Dai Y, Liu C, Zhou L, Ji X, Shi W-Q (2023) A large-scale screening of metal-organic frameworks for iodine capture combining molecular simulation and machine learning. Front Environ Sci Eng 17:148
He L, Chen L, Dong X, Zhang S, Zhang M, Dai X, Liu X, Lin P, Li K, Chen C, Pan T, Ma F, Chen J, Yuan M, Zhang Y, Chen L, Zhou R, Han Y, Chai Z, Wang S (2021) A nitrogen-rich covalent organic framework for simultaneous dynamic capture of iodine and methyl iodide. Chem 7:699–714
Geng K, He T, Liu R, Dalapati S, Tan KT, Li Z, Tao S, Gong Y, Jiang Q, Jiang D (2020) Covalent organic frameworks: Design, synthesis, and functions. Chem Rev 120:8814–8933
Allian AD, Takanabe K, Fujdala KL, Hao X, Truex TJ, Cai J, Buda C, Neurock M, Iglesia E (2011) Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J Am Chem Soc 133:4498–4517
Nakhaei Pour A, Karimi J, Keyvanloo Z, Hashemian M (2016) Size dependence adsorption of hydrogen on cobalt clusters: A DFT study. J Nano Res 42:100–111
Amaya-Roncancio S, Reinaudi L, Gimenez MC (2020) Adsorption and dissociation of CO on metal clusters. Mater Today Commun 24:101158
Al-Odail F, Mazher J (2022) A density functional theory study of adsorption and dissociation of H2 molecule on small PdnAgm (n + m ≤ 4) metal clusters. Struct Chem 34:1369–1383
Nanna ME, Bierwagen GP (2004) Mg-rich coatings: A new paradigm for Cr-free corrosion protection of Al aerospace alloys. J Coat Technol Res 1:69–80
Pollock TM (2010) Materials science. Weight loss with magnesium alloys. Science 328:986–987
Kim S-H, You B-S, Dong Yim C, Seo Y-M (2005) Texture and microstructure changes in asymmetrically hot rolled AZ31 magnesium alloy sheets. Mater Lett 59:3876–3880
Yoo J, Aksimentiev A (2011) Improved parametrization of Li+, Na+, K+, and Mg2+ Ions for All-Atom Molecular Dynamics Simulations of Nucleic Acid Systems. J Phys Chem Lett 3:45–50
Garrier S, Delhomme B, de Rango P, Marty P, Fruchart D, Miraglia S (2013) A new MgH2 tank concept using a phase-change material to store the heat of reaction. Int J Hydrogen Energy 38:9766–9771
Sadhasivam T, Kim H-T, Jung S, Roh S-H, Park J-H, Jung H-Y (2017) Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew Sust Energ Rev 72:523–534
Sun Y, Shen C, Lai Q, Liu W, Wang D-W, Aguey-Zinsou K-F (2018) Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater 10:168–198
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Delley B (2002) Hardness conserving semilocal pseudopotentials. Phys Rev B 66:155125
Halgren TA, Lipscomb WN (1977) The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett 49:225–232
Jia Y, Jiang J, Zheng R, Guo L, Yuan J, Zhang S, Gu M (2021) Insight into the reaction mechanism over PMoA for low temperature NH3-SCR: a combined In-situ DRIFTs and DFT transition state calculations. J Hazard Mater 412:125258
Liu J, Zhang X, Hu B, Lu Q, Liu D-J, Dong C-Q, Yang Y-P (2020) Formation mechanism of HCN and NH3 during indole pyrolysis: A theoretical DFT study. J Energy Inst 93:649–657
Jellinek J, Acioli PH (2002) Magnesium clusters: Structural and electronic properties and the size-induced nonmetal-to-metal transition. J Phys Chem A 106:10919–10925
Zeng T, He Y (2018) Scaling of the self-energy correction to the HOMO-LUMO gap with magnesium cluster size and its potential for extrapolating to larger magnesium clusters. J Appl Phys 124:044305
Maltsev AP, Charkin OP (2020) Theoretical Modeling of Stepwise Addition of H2 Molecules to Magnesium Clusters Mg18 and Mg17Ni. Russ J Org Chem 65:185–192
Bakhsh S, Liu X, Wang Y, He L, Ren X (2021) Beryllium and magnesium metal clusters: New globally stable structures and G0W0 calculations. J Phys Chem A 125:1424–1435
Akola J, Rytkönen K, Manninen M (2001) Metallic evolution of small magnesium clusters. Eur Phys J D 16:21–24
Balfour WJ, Douglas AE (1970) Absorption spectrum of the Mg2 molecule. Can J Phys 48:901–914
Janecek S, Krotscheck E, Liebrecht M, Wahl R (2011) Structure of Mgn and Mgn+ clusters up to n = 30. Eur Phys J D 63:377–390
Zhang J-M, Duan Y-N, Xu K-W, Ji V, Man Z-Y (2008) Ab initio calculation of neutral and singly charged Mgn (n⩽11) clusters. Physica B 403:3119–3124
Li C-M, Wu D, Tian X, Yu D, Li Y, Chen W (2021) Probing the effect of carbon do** on structures, properties, and stability of magnesium clusters. Theor Chem Acc 140:111
Shen D, Kong C-P, Jia R, Fu P, Zhang H-X (2015) Investigation of properties of Mgn clusters and their hydrogen storage mechanism: A study based on DFT and a global minimum optimization method. J Phys Chem A 119:3636–3643
Kumar V, Car R (1991) Structure, growth, and bonding nature of Mg clusters. Phys Rev B 44:8243
Thomas OC, Zheng W, Xu S, Bowen KH Jr (2002) Onset of metallic behavior in magnesium clusters. Phys Rev Lett 89:213403
**a X, Kuang X, Lu C, ** Y, **ng X, Merino G, Hermann A (2016) Deciphering the structural evolution and electronic properties of magnesium clusters: An aromatic homonuclear metal Mg17 cluster. J Phys Chem A 120:7947–7954
Zhao Y, Xu Y, Chen P, Yuan Y, Qian Y, Li Q (2021) Structural and electronic properties of medium-sized beryllium doped magnesium BeMg clusters and their anions. Res Phys 26:104341
Li K, Zhao Y-L, Deng J, He C-H, Ding S-J, Shi W-Q (2016) Adsorption of radioiodine on Cu2O surfaces: a first-principles density functional study. Acta Phys -Chim Sin 32:2264–2270
Brogan MA, Blake AJ, Wilson C, Gregory DH (2003) Magnesium diiodide, MgI2. Acta Crystallogr Sect C Cryst Struct Commun 59:i136–i138
Acknowledgements
The authors thank Prof. Huaqing Yang for providing the use of the Material Studio software.
Funding
This work was supported by the Natural Science Foundation of Sichuan Province (22NSFSC3451), the China West Normal University Cultivation Project (20A012), and the Urban & Green Technology Horizontal Technology Coordinating Office (UGT HTCO) Green Seed Fund (NIMR230801aACFUGT) by Agency for Science, Technology and Research (A*STAR).
Author information
Authors and Affiliations
Contributions
Na Wang: Conceptualization, Data Curation, Investigation, Formal Analysis, Writing - Original Draft. Jie Zhou: Conceptualization, Investigation, Formal Analysis, Writing - Original Draft. **angjun Kuang: Investigation, Methodology. Jianqi Qi: Resources, Funding Acquisition. Jun Zhou: Writing-review & editing, Funding Acquisition. Shijie Wang: Writing-review & editing. Tingting Song: Writing-review & editing, Resources, Funding Acquisition. Peng Sun: Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Zhou, J., Kuang, X. et al. Exploring the potential of magnesium clusters as effective adsorbents for gaseous radioactive iodine in nuclear energy applications. Struct Chem (2024). https://doi.org/10.1007/s11224-024-02346-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-024-02346-w