Abstract
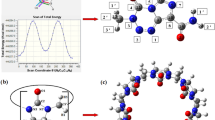
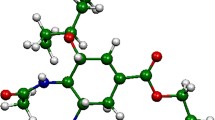
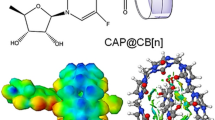
In this work, we have investigated the complex formation capability of gemcitabine drug with host cucurbit[n]urils, Q[n] (n = 6, 7, and 8) using density functional theory (DFT). The DFT studies demonstrate that the most stable configuration is a fully encapsulated complex. In the encapsulated gemcitabine@Q[6] and gemcitabine@Q[7] complexes, the gemcitabine amino -NH2 and the alcoholic groups bond with the carbonyl units of Q[n]. The addition of sodium ions leads to the partial exclusion of the gemcitabine molecule and the sodium atoms lie close to the carbonyl portal of Q[7]. Thermodynamic parameters computed for the complexation process exhibit high negative Gibbs free energy change, implying that the encapsulation process is spontaneous and is an enthalpy-driven process. Frontier molecular orbitals are located mainly on the gemcitabine uracil ring, before and after encapsulation, indicating that the encapsulation occurs by pure physical adsorption. Quantitative molecular electrostatic potentials demonstrate a shift in electron density during the complex formation and are more pronounced in gemcitabine@Q[7]. Atoms-in-molecule (AIM) topological analysis illustrate that these complexes are stabilized by various non-covalent interactions including hydrogen bonding (HBs) and C···F interactions. The reduced density gradient (RDG) graph exhibits the presence of strong HBs and weak van der Waals interactions and the presence of steric repulsion. The isosurface non-covalent interactions (NCI) diagram shows predominant steric interaction in the gemcitabine@Q[6] complex. The NCI isosurface for gemcitabine encapsulated complexes with Q[7] and Q[8] host displays that the green patches are uniformly distributed in all directions. Finally, EDA results demonstrate Pauling’s repulsive energy is predominant in gemcitabine@Q[6] complex, while the orbital and dispersion energies stabilize the gemcitabine@Q[7] complex.






Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the author on reasonable request.
Code availability
Not applicable.
References
Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K (2003) Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res 36:621–630
Barrow SJ, Kasera S, Rowland MJ, Del Barrio J, Scherman OA (2015) Cucurbituril-based molecular recognition. Chem Rev 115:12320–12406
Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K (2000) New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit [n] uril (n = 5, 7, and 8). J Am Chem Soc 122:540–541
Shetty D, Khedkar JK, Park KM, Kim K (2015) Can we beat the biotin–avidin pair?: Cucurbit[7]uril-based ultrahigh affinity host–guest complexes and their applications. Chem Soc Rev 44:8747–8761
Jansen K, Buschmann HJ, Wego A, Döpp D, Mayer C, Drexler HJ, Holdt HJ (2001) Schollmeyer, E.: Cucurbit[5]uril, decamethylcucurbit[5]uril and cucurbit[6]uril. Synthesis, solubility and amine complex formation. J Incl Phenom Macrocyclic Chem 39:357–363
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Christine Cripps M, Portenoy RK, Storniolo AM, Tarassoff P (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Veerman G, van Haperen VWT, Vermorken JB, Noordhuis P, Braakhuis BJ, Pinedo HM, Peters GJ (1996) Antitumor activity of prolonged as compared with bolus administration of 2′, 2′-difluorodeoxycytidine in vivo against murine colon tumors. Cancer Chemother Pharmacol 38:335–342
Veltkamp SA, Pluim D, van Eijndhoven MA, Bolijn MJ, Ong FH, Govindarajan R, Unadkat JD, Beijnen JH, Schellens JH (2008) New insights into the pharmacology and cytotoxicity of gemcitabine and 2′, 2′-difluorodeoxyuridine. Mol Cancer Ther 7:2415–2425
Laufer M, Ramalingam S, Schoenberg MP, Haisfield-Wolf ME, Zuhowski EG, Trueheart IN, Eisenberger MA, Nativ O, Egorin MJ (2003) Intravesical gemcitabine therapy for superficial transitional cell carcinoma of the bladder: a phase I and pharmacokinetic study. J Clin Oncol 21:697–703
Jelovac D, Armstrong DK (2011) Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 61:183–203
Stuurman FE, Nuijen B, Beijnen JH, Schellens JH (2013) Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacoknet 52:399–414
Oun R, Floriano RS, Isaacs L, Rowan EG, Wheate NJ (2014) The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol Res 3:447–455
Macartney DH (2011) Encapsulation of drug molecules by cucurbiturils: effects on their chemical properties in aqueous solution. Isr J Chem 51:600–615
Wheate NJ, Buck DP, Day AI, Collins JG (2006) Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans 451–458
Walker S, Oun R, McInnes FJ, Wheate NJ (2011) The potential of cucurbit[n]urils in drug delivery. Isr J Chem 51:616–624
Kemp S, Wheate NJ, Stootman FH, Aldrich-Wright JR (2007) The host-guest chemistry of proflavine with cucurbit[6, 7, 8]urils. Supramol Chem 19:475–484
Wu X, Zhang YM, Liu Y (2016) Nanosupramolecular assembly of amphiphilic guest mediated by cucurbituril for doxorubicin delivery. RSC Adv 6:99729–99734
Buczkowski A, Gorzkiewicz M, Stepniak A, Malinowska-Michalak M, Tokarz P, Urbaniak P, Ionov M, Klajnert-Maculewicz B, Palecz B (2020) Physicochemical and in vitro cytotoxicity studies of inclusion complex between gemcitabine and cucurbit[7]uril host. Bioorg Chem 99:103843
Buczkowski A, Palecz B, Schroeder G (2019) Stoichiometry and thermodynamics of gemcitabine and cucurbituril Q7 supramolecular complexes in high acidic aqueous solution. J Mol Struct 1178:554–563
Buczkowski A (2022) Thermodynamic study of pH and sodium chloride impact on gemcitabine binding to cucurbit[7]uril in aqueous solutions. J Mol Liq 345:117857
Sivalakshmidevi A, Om Reddy VG, Sivaram DVN, Swamy PV, Sairam RPP (2003) 2’-Deoxy-2’,2’-difluorocytidine monohydrochloride (gemcitabine hydrochloride). Acta Cryst E59:o1435–o1437
Venkataramanan NS, Suvitha A, Mizuseki H, Kawazoe Y (2015) Computational study on the interactions of mustard gas with cucurbituril macrocycles. Int J Quantum Chem 115:1515–1525
Grimme S, Antony J, Ehrlich S, Krieg H (2010) DFT-D3 – a dispersion correction for density functionals, Hartee-Fock and semi-empirical quantum chemical methods DFT-D3. J Chem Phys 132:154104
Sure R, Antony J, Grimme S (2014) Blind prediction of binding affinities for charged supramolecular host-guest systems: achievements and shortcomings of DFT-D3. J Phys Chem B 118:3431–3440
Assaf KI, Florea M, Antony J, Henriksen NM, Yin J, Hansen A, Qu Z-W, Sure R, Klapstein D, Gilson MK, Grimme S, Nau WM (2017) Hydrophobe challenge: a joint experiment and computational study on the host-guest binding of hydrocarbons to cucucurbiturils, allowing explicit evaluation of guest hydration free-energy contributions. J Phys Chem B 121:11144–11162
Suvitha A, Venkataramanan NS (2017) Trap** of organophosphorus chemical nerve agents by pillar[5]arene: a DFT, AIM, NCI and EDA analysis. J Incl Phenom Macrocyclic Chem 87:207–218
Klamt A (2018) The COSMO and COSMO-RS solvation models. Wiley Interdiscip Rev Comput Mol Sci 8:e1338
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T. Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery, Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin, RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2019) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford
Imane D, Leila N, Fatiha M, Abdelkrim G, Mouna C, Ismahan L, Abdelazize B (2020) Investigation of intermolecular interactions in inclusion complexes of pyroquilon with cucurbit[n]urils (n = 7, 8) using DFT-D3 correction dispersion. J Mol Liq 309:113233
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJA, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931
Ziegler T, Rauk A (1977) On the calculation of bonding energies by the Hartree Fock Slater method. I. The transition state method. Theor Chim Acta 46:1
AIMAll (Version 19.10.12), Keith TA, Gristmill TK: Software, Overland Park KS, USA, 2019 (aim.tkgristmill.com)
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Chemcraft - graphical software for visualization of quantum chemistry computations. https://www.chemcraftprog.com
Bardelang D, Udachin KA, Leek DM, Margeson JC, Chan G, Ratcliffe CI, Ripmeester JA (2011) Cucurbit[n]urils (n = 5 – 8): a comprehensive solid state study. Cryst Growth Des 11:5598–5614
Mecozzi S, Rebek J Jr (1998) The 55% solution: a formula for molecular recognition in the liquid state. Chem Eur J 4:1016–1022
Grimm LM, Spicher S, Tkachenko B, Scheiner PR, Grimme S, Biedermann F (2022) The role of packing dispersion, electrostatics, and solvation in high-affinity complexes of cucurbit[n]urils with uncharged polar guests. Chem Eur J 28:e202200529
Athare SV, Gejji SP (2018) Confinement of 1 butyl 3 methylimidazolium in cucurbiturils. J Mol Liq 272:496–506
Suvitha A, Venkataramanan NS, Sahara R, Kawazoe Y (2019) A theoretical exploration of the intermolecular interactions between resveratrol and water: a DFT and AIM analysis. J Mol Model 25:1–11
Venkataramanan NS, Suvitha A, Sahara R (2019) Structure, stability, and nature of bonding between high energy water clusters confined inside cucurbituril: a computational study. Comput Theor Chem 1148:44–54
Grishaeva TN, Masliy AN, Kuznetsov AM (2017) Water structuring inside the cavities of cucurbit[n]urils (n = 5 – 8): a quantum-chemical forecast. J Incl Pheom Marocyclic Chem 89:299–313
Masliy AN, Grishaeva TN, Kuznetsov AM (2018) Formation of aqua and tetrammine Cu(II) complexes inside the cavities of cucurbit[6,8]urils: a DFT forecast. Int J Quantum Chem 119:e25877
Biedermann F, Nau WM, Scheider H-J (2014) The hydrophobic effect revisited studies with supramolecular complexes imply high-energy water as a noncovalent driving forces. Angew Chem Int Ed 53:2–16
He S, Biedermann F, Vankova N, Zhechkov L, Heine T, Hoffman R, De Simone A, Duignan TT, Nau WM (2018) Cavitation energies can outperform dispersion interactions. Nat Chem 10:1252–1257
Grishaeva TN, Masliy AN, Kuznetsov AM (2022) The role of water molecular structuring in the formation of the inclusion compounds based on cucurbit[8]uril and trans-[Co(en)2 Cl2]+, trans-[Ru(en)2 Cl2]+ complexes: a DFT examination. J Incl Phenom Macrocyclic Chem 102:653–662
Venkataramanan NS, Suvitha A, Vijayaraghavan A, Thamotharan S (2017) Investigation of inclusion complexation of acetaminophen with pillar[5]arene: UV–Vis, NMR and quantum chemical study. J Mol Liq 241:782–791
Ma F, Zheng X, **e L, Li Z (2021) Sequence-dependent nanomolar binding of tripeptides containing N-terminal phenylalanine by cucurbit[7]uril: a theoretical study. J Mol Liq 328:115479
Aihara J-I (1999) Reduced HOMO-LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A 103:7487–7495
Imane D, Leila N, Fatiha M, Abdelkrim G, Mouna C, Ismahan L, Abdelazize B, Brahim H (2020) Investigation of intermolecular interactions in inclusion complexes of pyroquilon with cucurbit[n]urils (n = 7, 8) using DFT-D3 correction dispersion. J Mol Liq 309:113233
Venkataramanan NS, Suvitha A, Sahara R, Kawazoe Y (2021) A computational study on the complexation of bisbenzimidazolyl derivatives with cucurbituril and cyclohexylcucurbituril. J Incl Phenom Macrocyclic Chem 100:217–231
Yahiaoui K, Seridi L, Mansouri K (2021) Temozolomide binding to cucurbit[7]uril: QTAIM, NCI-RDG and NBO analyses. J Incl Phenom Macrocyclic Chem 99:61–77
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
Varadwaj A, Marques HM, Varadwaj PR (2019) Is the fluorine in molecules dispersive? Is molecular electrostatic potential a valid property to explore fluorine-centered non-covalent interactions? Molecules 24:379
Sarkar R, Kundu TK (2019) Nonbonding interaction analyses on PVDF/[BMIM][BF4] complex system in gas and solution phase. J Mol Model 25:1–27
Rozas I, Alkort I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Rozas I, Alkorta I, Elguero J (1998) Bifurcated hydrogen bonds: three-centered interactions. J Phy Chem A 102:9925–9932. https://doi.org/10.1021/jp9824813
Alkorta I, Elguero J (2004) Fluorine–fluorine interactions: NMR and AIM analysis. Struct Chem 15:117–120
López CS, Faza ON, Cossío FP, York DM, De Lera AR (2005) Ellipticity: a convenient tool to characterize electrocyclic reactions. Chem Eur J 11:1734–1738
Mahadevi AS, Sastry GN (2016) Cooperativity in noncovalent interactions. Chem Rev 116:2775–2825
Venkataramanan NS, Suvitha A, Kawazoe Y (2019) Unraveling the binding nature of hexane with quinone functionalized pillar[5]quinone: a computational study. J Incl Phenom Macrocyclic Chem 95:307–319
Suvitha A, Venkataramanan NS, Sahara R (2022) Effect of water molecule in the structure, stability, and electronic properties of sulfur trioxide clusters: a computational analysis. Monatsh Chem 153:347–357
Thellamurege N, Hirao H (2013) Water complexes of cytochrome P450: insights from energy decomposition analysis. Molecules 18:6782–6791
Acknowledgements
The author thanks the staff of the Center for Computational Materials Science, Institute for Materials Research, Tohoku University, and the supercomputer resources through the HPCI System Research Project (Project ID: hphp200040).
Author information
Authors and Affiliations
Contributions
AS carried out the formal analysis, conceiving the problem, and data curation for tables. NSV designed the methodology, supervised the work done, conceptualized the study, and edited the final manuscript. RS supervised the work done, conceptualized the study, and edited the final manuscript. YK supervised the work and edited the final manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Inclusion complexes are more stable.
• MESP analysis shows an electron density delocalization in the inclusion complex.
• Atoms in molecule analysis show the presence of hydrogen bonds and C···F interactions between host and gemcitabine.
• EDA shows a high Pauling’s repulsion for the inclusion complex with Q[6] as the host.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venkataramanan, N.S., Suvitha, A., Sahara, R. et al. Unveiling the gemcitabine drug complexation with cucurbit[n]urils (n = 6–8): a computational analysis. Struct Chem 34, 1869–1882 (2023). https://doi.org/10.1007/s11224-023-02133-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02133-z