Abstract
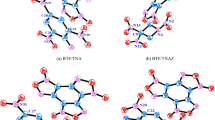
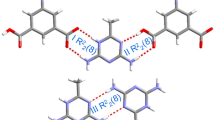
The structure, intermolecular interactions, electronic properties, and detonation performance of pure 3,6-Bis (1H-1,2,3,4tetrazol-5-ylamino)-s-tetrazine (BTATz) crystal and its three solvate cocrystals (solvents include 2-pyridone, DMF (N,N-dimethyl-formamide), and pyrazine) were studied using density functional theory and molecular dynamics. It is found that moderate N–H∙∙∙O or N–H∙∙∙N hydrogen bonds are the main forces for stabilizing the BTATz-based crystals. The BTATz/pyrazine cocrystal with face-face configuration, caused by big π-π conjugation, hydrogen bonds, and widespread vdW interactions, has the lowest impact sensitivity among the three solvate-cocrystals. Relatively, strong electron delocalization of H in BTATz induced by the pyrazine results in strong N–H∙∙∙N interactions in the BTATz/pyrazine cocrystal. Crystal packing types and hydrogen bonds affect the stability of the BTATz-based cocrystals. Our work may provide an insight into the property variation of the BTATz-based cocrystals under the influence of different solvents and is helpful for the design of new energetic solvate-cocrystals.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Saikia A, Sivabalan R, Polke BG, Gore GM, Singh A, Rao AS, Sikder AK (2009) Synthesis and characterization of 3,6-bis(1H–1,2,3,4-tetrazol-5-ylamino)-1,2,4,5-tetrazine (BTATz): novel high-nitrogen content insensitive high energy material. J Hazard Mater 170:306–313
Anniyappan M, Talawar MB, Sinha RK, Murthy KPS (2020) Review on advanced energetic materials for insensitive munition formulations. Combust, Explo, Shock+ 56(5):495–519
Zhang CY, Jiao FB, Li HZ (2018) Crystal engineering for creating low sensitivity and highly energetic materials. Cryst Growth Des 18(10):5713–5726
Jiao FB, **ong Y, Li HZ, Zhang CY (2018) Alleviating the energy & safety contradiction to construct new low sensitivity and highly energetic materials through crystal engineering. CrystEngComm 20:1757–1768
Bu RP, Jiao FB, Liu GR, Zhao JY, Zhang CY (2020) Categorizing and understanding energetic crystals. Cryst Growth Des 21(1):3–15
Bedard M, Huber H, Myers JL, Wright GF (1962) The crystalline form of 1,3,5,7-tetranitro-1,3,5,7-tetrazacyclooctane (HMX). Candian J of Chem 40(12):2278–2299
Wu Q, Li MQ, Hu QN, Zhang ZW, Zhu WH (2020) First-principle study and Hirshfeld surface analysis on the effect of H2O, NH3 and H2S on structural, electronic, elastic, optical and thermodynamic properties of a novel high-energy crystal 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide. J Mater Sci 55:237–249
Zhang JH, Shreeve JM (2016) Time for pairing: cocrystals as advanced energetic materials. CrystEngComm 18:6124–6133
Landenberger KB, Matzger AJ (2010) Cocrystal engineering of a prototype energetic material supramolecular chemistry of 2,4,6-trinitrotoluene. Cryst Growth Des 10:5341–5347
Bolton O, Matzger AJ (2011) Improved stability and smart-material functionality realized in an energetic cocrystal. Angew Chem Int Edit 50:8960–8963
Bolton O, Simke LR, Pagoria PF, Matzger AJ (2012) High power explosive with good sensitivity: a 2:1 cocrystal of CL-20:HMX. Cryst Growth Des 12:4311–4314
Landenberger KB, Bolton O, Matzger AJ (2015) Energetic-energetic cocrystals of diacetone diperoxide (DADP): dramatic and divergent sensitivity modifications via cocrystallization. J Am Chem Soc 137:5074–5079
Landenberger KB, Bolton O, Matzger AJ (2013) Two isostructural explosive cocrystals with significantly different thermodynamic stabilities. Angew Chem Int Edit 52:6468–6471
Liu GR, Bu RP, Huang X, Zhong K, Jiao FB, Wei SH, Li HZ, Zhang CY (2022) Energetic cocrystallization as the most significant crystal engineering way to create new energetic materials. Cryst Growth Des 22:954–970
Selig W (1982) New adducts of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). Propel, Explo, Pyro 7:70–77
Selig W (1982) New adducts of 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX). Propel, Explo, Pyro 6:1–4
Liu Y, Li SC, Xu JJ, Zhang HL, Guan YX, Jiang HY, Huang SL, Huang H, Wang ZS (2018) Three energetic 2,2 ’,4,4 ’,6,6 ’-hexanitrostilbene cocrystals regularly constructed by H-bonding, pi-stacking, and van der Waals interactions. Cryst Growth Des 18:1940–1943
Aakeroy CB, Wijethunga TK, Desper J (2015) Crystal engineering of energetic materials: co-crystals of ethylenedinitramine (EDNA) with modified performance and improved chemical stability. Chem-Eur J 21:11029–11037
Liu GR, Wei SH, Zhang CY (2020) Review of the intermolecular interactions in energetic molecular cocrystals. Cryst Growth Des 20:7065–7079
Zhao X, Zhu W (2022) Recent advances in studying the nonnegligible role of noncovalent interactions in various types of energetic molecular crystals. CrystEngComm 24:6119–6136
Fedyanin IV, Lyssenko KA, Fershtat LL, Muravyev NV, Makhova NN (2019) Crystal solvates of energetic 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane molecule with [bmim]-based ionic liquids. Cryst Growth Des 19:3660–3669
Fondren NS, Fondren ZT, Unruh DK, Maiti A, Cozzolino A, Lee YJ, Hope-Weeks L, Weeks B (2020) Study of physicochemical and explosive properties of a 2,4,6-trinitrotoluene/aniline cocrystal solvate. Cryst Growth Des 20(1):116–129
Loschen C, Klamt A (2015) Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering. J Pharm Pharmacol 67:803–811
Chapman CJ, Groven LJ (2021) Evaluation of solvate and co-crystal screening methods for CL-20 containing energetic materials. J of Ener Mater 40:258–272
Landenberger KB, Matzger AJ (2012) Cocrystals of 1,3,5,7-tetranitro-1,3,5,7-tetrazacyclooctane (HMX). Cryst Growth Des 12:3603–3609
Wang K, Zhu WH (2021) Computational insights into the formation driving force of CL-20 based solvates and their desolvation process. CrystEngComm 23:2150–2161
Chavez DE, Hiskey MA, Naud DL (2004) Tetrazine explosives. Propel, Explo, Pyro 29:209–215
Zhang ZB, Li T, Yin L, Yin X, Zhang JG (2016) A novel insensitive cocrystal explosive BTO/ATZ: preparation and performance. RSC Adv 6:76075–76083
Son SF, Berghout HL, Bolme CA, Chavez DE, Naud D, Hiskey MA (2000) Burn rate measurements of HMX, TATB, DHT, DAAF, and BTATz. Proc of Combust Inst 28:919–924
Kent RV, Wiscons RA, Sharon P, Grinstein D, Frimer AA, Matzger AJ (2017) Cocrystal engineering of a high nitrogen energetic material. Cryst Growth Des 18:219–224
Segall MD, Lindan PJD, Lindan MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) First-principles simulation: ideas, illustrations and the CASTEP code. J Phys: Condensed Matter 14:2717–2744
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Keshavarz MH (2007) Reliable estimation of performance of explosives without considering their heat contents. J Hazard Mater 147:826–831
Keshavarz MH (2010) Simple relationship for predicting impact sensitivity of nitroaromatics, nitramines, and nitroaliphatics. Propel, Explo, Pyro 35:175–181
Spackman AMJD (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Lu T, Chen FW (2012) Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J Mol Graphics Modell 38:314–323
Lu T, Chen Q (2020) A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor Chem Acc 139:25–37
Emamian S, Lu T, Kruse H, Emamian H (2019) Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J Comput Chem 40:2868–2881
Shishkina AV, Zhurov VV, Stash AI, Vener MV, Pinkerton AA, Tsirelson VG (2013) Noncovalent interactions in crystalline picolinic acid n-oxide: insights from experimental and theoretical charge density analysis. Cryst Growth Des 13:816–828
Bu RP, **ong Y, Zhang CY (2020) π–π stacking contributing to the low or reduced impact sensitivity of energetic materials. Cryst Growth Des 20:2824–2841
Tian BB, **ong Y, Chen LZ, Zhang CY (2018) Relationship between the crystal packing and impact sensitivity of energetic materials. CrystEngComm 20:837–848
Stalke D (2011) Meaningful structural descriptors from charge density. Chem-Eur J 17:9264–9278
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang WT (2010) Reavealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Kretic DS, Radovanovic JI, Veljkovic DZ (2021) Can the sensitivity of energetic materials be tuned by using hydrogen bonds? Another look at the role of hydrogen bonding in the design of high energetic compounds. Phys Chem Chem Phys 23:7472–7479
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Defu Wang and Kun Wang. The first draft of the manuscript was written by Defu Wang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Wang, K. & Zhu, W. Theoretical insights into the roles of intermolecular interactions in BTATz-based solvate cocrystals. Struct Chem 34, 1685–1697 (2023). https://doi.org/10.1007/s11224-022-02084-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02084-x