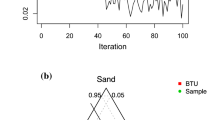
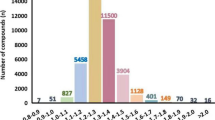
Abstract
Atom-pairs proportions are the transparent quality of a molecule: if a molecule has two atoms of oxygen and three atoms of nitrogen, the atom-pair atom1-atom2 can be expressed as a code “atom1-atom2-n1-n2,” indicating the different atoms and their numbers. These codes for a group of atoms (nitrogen, oxygen, sulfur, phosphorus, fluorine, chlorine, bromine, as well as double and triple covalent bonds) are applied to build up the so-called optimal molecular descriptor calculated with special coefficients named correlation weights of corresponding pairs. The numerical data on the correlation weights are calculated by the Monte Carlo technique using the CORAL software (http://www.insilico.eu/coral). The one-variable model for melting points of 8653 different organic compounds is characterized by the following statistical quality: n=6483, r2=0.6452, RMSE=61.9°C; n=2170, r2=0.7941, RMSE=39.2°C.
Similar content being viewed by others
Data availability
Data are available within the article or its supplementary materials.
Code availability
CORAL software (http://www.insilico.eu/coral)
References
Tetko IV, Sushko Y, Novotarskyi S, Patiny L, Kondratov I, Petrenko AE, Charochkina L, Asiri AM (2014) How accurately can we predict the melting points of drug-like compounds? J Chem Inf Model 54(12):3320–3329. https://doi.org/10.1021/ci5005288
Tetko IV, Yan A, Gasteiger J (2018) Prediction of physicochemical properties of compounds. In: Engel T, Gasteiger J (eds) Applied Chemoinformatics, Chapter 3, pp 53–81. https://doi.org/10.1002/9783527806539.ch3
Yan F, Shi Y, Wang Y, Jia Q, Wang Q, **a S (2020) QSPR models for the properties of ionic liquids at variable temperatures based on norm descriptors. Chem Eng Sci 217:115540. https://doi.org/10.1016/j.ces.2020.115540
ten Berge W (2009) A simple dermal absorption model: derivation and application. Chemosphere 75(11):1440–1445. https://doi.org/10.1016/j.chemosphere.2009.02.043
Dearden JC (1991) The QSAR prediction of melting point, a property of environmental relevance. Sci Total Environ 109–110:59–68. https://doi.org/10.1016/0048-9697(91)90170-J
Dearden JC (2003) Quantitative structure-property relationships for prediction of boiling point, vapor pressure, and melting point. Environ Toxicol Chem 22:1696–1709. https://doi.org/10.1897/01-363
Brown TN, Armitage JM, Arnot JA (2019) Application of an iterative fragment selection (IFS) method to estimate entropies of fusion and melting points of organic chemicals. Mol Inform 38(8-9):1800160. https://doi.org/10.1002/minf.201800160
McDonagh JL, Van Mourik T, Mitchell JBO (2015) Predicting melting points of organic molecules: applications to aqueous solubility prediction using the general solubility equation. Mol Inform 34(11-12):715–724. https://doi.org/10.1002/minf.201500052
Venkatraman V, Evjen S, Knuutila HK, Fiksdahl A, Alsberg BK (2018) Predicting ionic liquid melting points using machine learning. J Mol Liq 264:318–326. https://doi.org/10.1016/j.molliq.2018.03.090
Fatemi MH, Izadian P (2012) In silico prediction of melting points of ionic liquids by using multilayer perceptron neural networks. J Theor Comput Chem 11(1):127–141. https://doi.org/10.1142/S0219633612500083
Park HY, Li J, Park B-H, Kim CK (2015) MSEP and CoMFA studies on the melting points of nitroaromatic compounds. Bull Kor Chem Soc 36(7):1838–1847. https://doi.org/10.1002/bkcs.10356
Hemmateenejad B, Shamsipur M, Zare-Shahabadi V, Akhond M (2011) Building optimal regression tree by ant colony system-genetic algorithm: Application to modeling of melting points. Anal Chim Acta 704(1-2):57–62. https://doi.org/10.1016/j.aca.2011.08.010
Bhat AU, Merchant SS, Bhagwat SS (2008) Prediction of melting points of organic compounds using extreme learning machines. Ind Eng Chem Res 47(3):920–925. https://doi.org/10.1021/ie0704647
Kang J-W, Kwon OK, Lee S, Lee SH, Kim DH, Hwang H-J (2010) Kinetic lattice Monte Carlo simulations of vacancy diffusion in silicon below the melting point. J Comput Theor Nanosci 7(3):604–611. https://doi.org/10.1166/jctn.2010.1401
Karthikeyan M, Glen RC, Bender A (2005) General melting point prediction based on a diverse compound data set and artificial neural networks. Chem Inf Model 45(3):581–590. https://doi.org/10.1021/ci0500132
Toropova AP, Toropov AA (2014) CORAL software: prediction of carcinogenicity of drugs by means of the Monte Carlo method. Eur J Pharm Sci 52(1):21–25. https://doi.org/10.1016/j.ejps.2013.10.005
Garro Martinez JC, Duchowicz PR, Estrada MR, Zamarbide GN, Castro EA (2011) QSAR study and molecular design of open-chain enaminones as anticonvulsant agents. Int J Mol Sci 12(12):9354–9368. https://doi.org/10.3390/ijms12129354
Achary PGR (2014) Simplified molecular input line entry system-based optimal descriptors: QSAR modelling for voltage-gated potassium channel subunit Kv7.2. SAR QSAR Environ Res 25(1):73–90. https://doi.org/10.1080/1062936X.2013.842930
Toropov AA, Toropova AP, Carnesecchi E, Benfenati E, Dorne JL (2020) The index of ideality of correlation and the variety of molecular rings as a base to improve model of HIV-1 protease inhibitors activity. Struct Chem 31:1441–1448. https://doi.org/10.1007/s11224-020-01525-9
Toropov AA, Toropova AP, Veselinović AM, Leszczynska D, Leszczynski J (2020) SARS-CoV Mpro inhibitory activity of aromatic disulfide compounds: QSAR model. J Biomol Struct Dyn Published online: 09 Sep 2020. https://doi.org/10.1080/07391102.2020.1818627
Rescifina A, Floresta G, Marrazzo A, Parenti C, Prezzavento O, Nastasi G, Dichiara M, Amata E (2017) Development of a Sigma-2 receptor affinity filter through a Monte Carlo based QSAR analysis. Eur J Pharm Sci 106:94–101. https://doi.org/10.1016/j.ejps.2017.05.061
Veselinović AM, Veselinović JB, Živković JV, Nikolić GM (2015) Application of smiles notation based optimal descriptors in drug discovery and design. Curr Top Med Chem 15(18):1768–1779. https://doi.org/10.2174/1568026615666150506151533
Toropov AA, Toropova AP (2021) Quasi-SMILES as a basis for the development of models for the toxicity of ZnO nanoparticles. Sci Total Environ 772:145532. https://doi.org/10.1016/j.scitotenv.2021.145532
Mansouri K, Grulke CM, Judson RS, Williams AJ (2018) OPERA models for predicting physicochemical properties and environmental fate endpoints. Aust J Chem 10(1):10. https://doi.org/10.1186/s13321-018-0263-1
Toropov AA, Toropova AP (2017) The index of ideality of correlation: a criterion of predictive potential of QSPR/QSAR models? Mutat Res 819:31–37. https://doi.org/10.1016/j.mrgentox.2017.05.008
Toropova AP, Toropov AA (2019) Does the index of ideality of correlation detect the better model correctly? Mol Inform 38(8-9):1800157. https://doi.org/10.1002/minf.201800157
Watkins M, Sizochenko N, Rasulev B, Leszczynski J (2016) Estimation of melting points of large set of persistent organic pollutants utilizing QSPR approach. J Mol Model 22(3):1–14. https://doi.org/10.1007/s00894-016-2917-0
Funding
The authors are grateful for the contribution of the project LIFE-CONCERT (LIFE17 GIE/IT/000461) for the financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: A. P. T., A. A. T., and E. B.
Methodology: A. P. T., A. A. T., and E. B.
Software: A. A. T..
Validation: A. P. T., A. A. T., and E. B.
Formal analysis: A. P. T.
Data curation: A. P. T. and A. A. T.
Writing—original draft preparation: A. P. T. and A. A. T.
Writing—review and editing: A. P. T., A. A. T., and E. B.
Supervision: E. B.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 275 kb)
Rights and permissions
About this article
Cite this article
Toropova, A.P., Toropov, A.A. & Benfenati, E. The self-organizing vector of atom-pairs proportions: use to develop models for melting points. Struct Chem 32, 967–971 (2021). https://doi.org/10.1007/s11224-021-01778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01778-y