Abstract
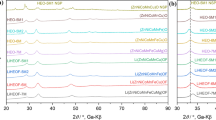
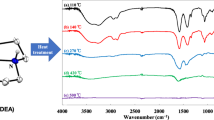
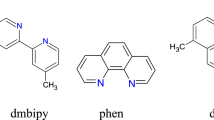
A new series of lanthanide complexes [Ln(3,4-DMBA)3phen]2 [Ln(III) = Nd(1), Sm(2), Tb(3) and Dy(4), 3,4-DMBA = 3,4-dimethylbenzoate, phen = 1,10-phenanthroline] have been synthesized and characterized by elemental analysis, infrared spectra and TG-DTG techniques. The single crystals of the complexes 3 and 4 have been obtained and their structures have been determined by single-crystal X-ray diffraction. In the complexes 3 and 4, each Ln(III) ion is coordinated by four bidentate-bridging 3,4-DMBA ligands, one bidentate-chelating 3,4-DMBA group and one bidentate-chelating phen ligand, giving a coordination number of eight. The complex 3 shows bright green luminescence under ultraviolet light in the solid state. Thermal analysis of the complexes 1–4 are discussed by TG-DTG and IR techniques. The non-isothermal kinetics of the complexes 1–4 are investigated by using double equal-double step method. The thermodynamic parameters (ΔH ≠, ΔG ≠ and ΔS ≠) and kinetic parameters (activation energy E and the pre-exponential factor A) of the four complexes are also calculated.









Similar content being viewed by others
References
Wang RF, ** LP, Wang MZ, Huang SH, Chen XT (1995) Acta Chim Sin 53:39–45
Zhang Y, ** LP, Lŭ SZ (1997) J Inorg Chem 13:280–287 (in Chinese)
Deng H, Cai YP, Chao H (2003) Chin J Inorg Chem 21:409–414
Li X, Zhang ZY, Zou YQ (2005) Eur J Inorg Chem 2005:2909–2918
Chen Y, Cai WM (2005) Spectrochim Acta A 62:863–868
Yan CH, Guo CF, Lu P, Zhang M, Qiu GM (2007) J Rare Earth 25:117–121
Fu YL, Zhang JC, Lv YG, Cao WL (2008) Spectrochim Acta A 70:646–650
Huang Y, Yan B, Shao M (2009) J Solid State Chem 182:657–668
Yang J, Wang JC, Liu XS, Wu YP, He Zhang, Wei Zheng (2007) J Chinese Rare Earth Soc 25:78–81 (in Chinese)
Kang JG, Kim TJ, Kang HJ, Park Y, Nah MK (2008) J Lumin 128:1867–1872
Cui HX, Chen JM, Zhou HD, Lu YH (2007) Spectrochim Acta A 68:478–483
Irfanullah M, Iftikhar K (2009) Inorg Chem Commun 12:296–299
Dang S, Sun LN, Song SY, Zhang HJ, Zheng GL, Bi YF, Guo HD, Guo ZY, Feng J (2008) Inorg Chem Commun 11:531–534
Shi YZ, Sun XZ, Jiang YH (1988) Spectra and chemical identification of organic compounds, Science and Technology Press, Nan**g, p 98
Xu CJ, **e F, Guo XZ, Yang H (2005) Spectrochim Acta A 61:2005–2008
Yin MC, Ai CC, Yuan LJ, Wang CW, Sun JT (2004) J Mol Struct 691:33–37
Lam AWH, Wong WT, Gao S, Wen GH, Zhang XX (2003) Eur J Inorg Chem 2003:149–163
Wang LY, Zheng XJ, ** LP (1999) Chem J Chin Univ 20:1110–1114
Starink MJ (2003) Thermochim Acta 404:163–176
Lu ZR, Ding YC, Xu Y, Li BL, Zhang Y (2005) J Inorg Chem 21:181–185 (in Chinese)
Zhang JJ, Ren N (2004) Chin J Chem 22:1459–1462
Gao Z, Nakada M, Amasski I (2001) Thermaochim Acta 369:137–142
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ (2008) Thermal analysis kinetics, 2nd edn. Science Press, Bei**g, p 151
Straszko J, Olstak-Humienik M, Mozejko J (1997) Thermochim Acta 292:145–150
Olstak-Humienik M, Mozejko J (2000) Thermochim Acta 344:73–79
Acknowledgements
This project was supported by the National Natural Science Foundation of China (nos. 20773034 and 20601007) and the Natural Science Foundation of Hebei Province (no. B2007000237).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, HM., Ren, N., Zhang, JJ. et al. Synthesis, crystal structures and thermal decomposition kinetics of four new lanthanide complexes with 3,4-dimethylbenzoic acid and 1,10-phenanthroline. Struct Chem 21, 165–173 (2010). https://doi.org/10.1007/s11224-009-9555-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9555-4