Abstract
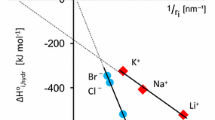
The results of calculations of the hydration free energy for monovalent ions by the in- tegral equation method within the framework of the RISM approach with different sets of parameters of the Lennard-Jones potential are presented. The main goal of the study is to choose the optimum potential model providing reliable and the most accurate description of ion hydration thermodynamics and the fit to experimental data. The accuracy of calculations of the hydration free energy was tested using five equations available in the literature and five sets of parameters of the Lennard-Jones potential. The proper choice of parameters of the Lennard-Jones potential plays a significant role in RISM calculations of thermodynamic prop- erties, whereas the use of improved models in calculations of the hydration free energy does not always lead to more accurate results
Similar content being viewed by others
References
F. Hirata, Molecular Theory of Solvation, Kluwer Acad. Publ., Dordrecht, 2003, 360 pp.
G. N. Chuev, M. V. Bazilevskii, Usp. Khim., 2003, 72, 827 [Russ. Chem. Rev. (Engl. Transl.), 2003, 72, 735].
D. Chandler, H. C. Andersen, J. Chem. Phys., 1972, 57, 1930.
M. V. Fedotova, M. F. Golovko, Metod integralńykh uravne- nii v ravnovesnoi statisticheskoi teorii zhidkostei [Integral Equa- tion Method in Equilibrium Statistical Theory of Liquids], in Teoreticheskie i eksperimentalńye metody khimii rastvorov [Theoretical and Experimental Methods of Solution Chemis- try], Prospekt, Moscow, 2011 (in Russian).
C. G. Gray, K. E. Gubbins, Theory of Molecular Fluids, Clarendon Press, Oxford, 1985, 626 pp.
J. P. Hansen, I. R. McDonald, Theory of Simple Liquids, 3rd ed., Acad. Press, New York, 2006, 410 pp.
J. Aqvist, J. Phys. Chem., 1990, 94, 8021.
J. Chandrasekhar, D. C. Spellmeyer, W. L. Jorgensen, J. Am. Chem. Soc., 1984, 106, 903.
W. L. Jorgensen, J. Tirado-Rives, J. Am. Chem. Soc., 1988, 110, 1657.
N. A. McDonald, E. M. Duffy, W. L. Jorgensen, J. Am. Chem. Soc., 1998, 120, 5104.
K. P. Jensen, W. L. Jorgensen, J. Chem. Theory Comput., 2006, 2, 1499.
L. X. Dang, Chem. Phys. Lett., 1992, 200, 21.
L. X. Dang, J. Am. Chem. Soc., 1995, 117, 6954.
L. X. Dang, B. C. Garret, J. Chem. Phys., 1993, 99, 2972.
L. X. Dang, J. Chem. Phys., 1992, 96, 6970.
L. X. Dang, J. Chem. Phys., 1995, 102, 3483.
I. S. Joung, Th. E. Cheatham III, J. Phys. Chem. B, 2008, 112, 9020.
D. Horinek, Sh. I. Mamatkulov, R. R. Netz, J. Chem. Phys., 2009, 130, 124507.
S. J. Singer, D. Chandler, Mol. Phys., 1981, 55, 621.
A. Kovalenko, F. Hirata, J. Chem. Phys., 2000, 113, 2793.
D. Chandler, Y. Singh, D. Richardson, J. Chem. Phys., 1984, 81, 1975.
S. Ten-no, J. Chem. Phys., 2001, 115, 3724.
G. N. Chuev, M. V. Fedorov, J. Crain, Chem. Phys. Lett., 2007, 448, 198.
P. A. Monson, G. P. Morris, in Adv. Chem. Phys., Eds I. Prigogine, S. A. Rice, John Wiley and Sons, Hoboken (NJ), 1990, 77, 451.
T. Ichiye, D. Chandler, J. Phys. Chem., 1988, 92, 5257.
L. Lue, D. Blankschtein, J. Phys. Chem., 1992, 96, 8582.
H.-A. Yu, B. Roux, M. Karplus, J. Chem. Phys., 1990, 92, 5020.
S. H. Chong, F. Hirata, J. Phys. Chem. B, 1997, 101, 3209.
M. V. Fedorov, A. A. Kornyshev, Mol. Phys., 2007, 105, 1.
G. N. Chuev, S. E. Erofeeva, V. F. Sokolov, Biofizika, 2007, 52, 773 [Biophysics (in Russian), 2007, 52, 773].
G. N. Chuev, M. V. Fedorov, S. Chiodo, N. Russo, E. Sicilia, J. Comput. Chem., 2008, 29, 2406.
H. J. C. Berendsen, J. R. Grigera, T. P. Straatsma, J. Phys. Chem., 1987, 91, 6269.
G. N. Chuev, M. V. Fedorov, J. Comput. Chem., 2004, 25, 1369.
W. L. Jorgensen, D. S. Maxwell, J. Tirado-Rives, J. Am. Chem. Soc., 1996, 117, 11225.
W. Hackbusch, Multi-Grid Methods and Applications, Spring- er-Verlag, Berlin, 1985, 377 pp.
M. V. Fedorov, W. Hackbusch, Preprint Max-Plank-Institut fur Mathematik in den Naturwissenschaften, Leipzig, 2008, 11 p., http://www.mis.mpg.de/preprints/2008/preprint-2008-88.pdf.
S. H. Lee, J. C. Rasaiah, J. Phys. Chem., 1996, 100, 1420.
S. Koneshan, J. C. Rasaiah, R. M. Lynden-Bell, S. H. Lee, J. Phys. Chem. B, 1998, 102, 4193.
J. Florian, A. Warshel, J. Phys. Chem. B, 1999, 103, 10282.
J. R. Pliego Jr., J. M. Riveros, Chem. Phys. Lett., 2000, 332, 597.
B. M. Pettitt, P. J. Rossky, J. Chem. Phys., 1986, 84, 5836.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 218–223, February, 2011.
Rights and permissions
About this article
Cite this article
Fedotova, M.V., Kruchinin, S.E. On calculations of the ion hydration free energy within the framework of the RISM approach. Russ Chem Bull 60, 223–228 (2011). https://doi.org/10.1007/s11172-011-0037-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-011-0037-7