Abstract
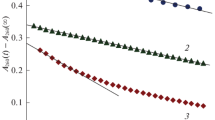
The influence of oxygen, hydrogen peroxide, and phosphate and carbonate ions on the kinetics of ozone decomposition in an aqueous solutions was studied by using mathematical simulation. The apparent rate constants of ozone decomposition and the hydroxyl radical concentration in the presence of compounds accelerating and inhibiting the ozone decomposition process were calculated. The calculated data were compared with the experimental results taken from literature sources.
Similar content being viewed by others
References
A. N. Ignat’ev, A. N. Pryakhin, V. V. Lunin, Izv. Akad. Nauk, Ser. Khim., 2008, 6, 1151 [Russ. Chem. Bull., Int. Ed., 2008, 57, 1172].
K. Sehested, H. Cortfitzen, J. Holcman, C. Fischer, E. Hart, Env. Sci. Tech., 1991, 25, 1589.
B. Bezbarua, D. Reckhow, Ozone: Sci. Eng., 2004, 26, 345.
H. Tomiyasu, H. Fukutomi, G. Gordon, Inorg. Chem., 1985, 24, 2962.
A. Nemes, I. Fabian, R. Eldik, J. Phys. Chem. A., 2000, 104, 7995.
A. Nemes, I. Fabian, G. Gordon, Ozone: Sci. Eng., 2000, 22, 287.
J. Duguet, Ozone: Sci. Eng., 1985, 7, 241.
I. Fabian, Prog. Nucl. Energy, 1995, 29, 167.
Notre Dame Radiation laboratory data center http://www.rcdc.nd.edu/browse-compil.html.
J. Acero, U. Gunten, Ozone: Sci. Eng., 2000, 22, 305.
L. Fornl, D. Bahnemann, E. Hart, J. Phys. Chem., 1982, 86, 255.
T. Mizuno, H. Tsuno, H. Yamada, Ozone: Sci. Eng., 2007, 29, 31.
P. Maruthamuthu, D. Neta, J. Phys. Chem., 1977, 81, 1622.
P. Maruthamuthu, D. Neta, J. Phys. Chem., 1978, 82, 710.
R. Buhler, J. Staehelin, J. Hoigne, J. Phys. Chem., 1984, 88, 2560.
M. Zenaitis, S. Duff, Ozone: Sci. Eng., 2005, 27, 397.
T. Mizuno, H. Tsuno, H. Yamada, Ozone: Sci. Eng., 2007, 29, 55.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1069–1077, June, 2009.
Rights and permissions
About this article
Cite this article
Ignat’ev, A.N., Pryakhin, A.N. & Lunin, V.V. Mathematical simulation as a method to study the influence of oxygen, hydrogen peroxide, and phosphate and carbonate ions on the kinetics of ozone decomposition in aqueous solution. Russ Chem Bull 58, 1097–1105 (2009). https://doi.org/10.1007/s11172-009-0142-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-009-0142-z