Abstract
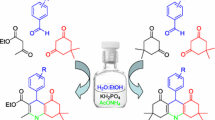
In this work, we explored the convenient and sustainable method for the synthesis of 1, 8 dioxodecahydroacridine derivatives was achieved via a one-pot condensation reaction of dimedone, para-nitrobenzaldehyde, and ammonium acetate by using theophylline as a catalyst at room temperature in aqueous medium. This environmentally friendly method boasts several notable features, including high product yields, rapid completion of reactions, the use of eco-friendly and bio-based catalysts, straightforward work-up procedures without the need for column chromatography, cost-effectiveness, clean synthesis practices that avoid the use of harmful organic solvents, and exceptional atom efficiency.








Similar content being viewed by others
Data availability
Data will be made available on request.
References
G. Vasuki, K. Kumaravel, Tetrahedron Lett. 49, 5636–5638 (2008)
M.K. Kenarl, S. Asgharl, B. Malekl et al., Res. Chem. Intermed. (2023). https://doi.org/10.1007/s11164-023-05165-6
H. Atharifar, A. Keivanloo, B. Maleki et al., Res. Chem. Intermed. (2023). https://doi.org/10.1007/s11164-023-05152-x
A. Sapkal, S. Attar, A. Kadam et al., Polycycl. Aromat. Compd. 43, 7719–7731 (2023)
A. Sapkal, S. Kamble, J. Heterocycl. Chem. 57, 3597–3604 (2020)
S.K. Tiwari, K.N. Shivhare, M.K. Patel et al., Polycycl. Aromat. Compd. 42, 1035–1047 (2022)
D. Verma, V. Sharma, S. Jain et al., J. Dispers. Sci. Technol. 41, 1145–1158 (2020)
W.A. Denny, Curr. Med. Chem. 9, 1655–1665 (2022)
M. Demeunynck, F. Charmantray, A. Martelli, Curr. Pharm. Des. 7, 1703–1724 (2001)
M.O. Anderson et al., Bioorg. Med. Chem. 14, 334–343 (2006)
Y. Mikata, M. Chikira, S. Yano et al., Inorgan. Chem. Acta. 279, 51–57 (1998)
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy et al., J. Mol. Catal. 247, 233–239 (2006)
S.M. Vahdat et al., Arab. J. Chem. 12, 1515–1521 (2019)
B. Maleki, R. Tayebee, M. Kermanian et al., J. Mex. Chem. 57, 290–297 (2013)
N. Madankumar, K. Pitchumani, Chem. Select 3, 10886–10891 (2018)
B. Maleki, H. Atharifar, O. Reiser et al., Polycycl. Aromat. Compd. 41, 721–734 (2019)
M.M. Alam, A.T. Mubarak, M.A. Assiri et al., BMC Chem. 13, 1–10 (2019)
A. Nakhaei, A. Davoodnia, A. Morsali, Res. Chem. Intermed. 41, 7815–7826 (2015)
A. Davoodnia, A. Zare-Bidaki, H. Behmadi, Chin. J. Catal. 33, 1797–1801 (2012)
R. Rezaei, R. Khalifeh, M. Rajabzadeh et al., Heterocycl. Comm. 19, 57–63 (2013)
S.R. Mousavi, H.R. Nodeh, A. Foroumadi, Polycycl. Aromat. Compd. 41, 746–760 (2021)
M.N. Esfahani, Z. Rafiee, H. Kashi, J. Iran. Chem. Soc. 13, 1449–1461 (2016)
M. Mazloumi, F. Shirini, J. Mol. Struct. 1217, 128326 (2020)
M. Kiani, M. Mohammadipour, RSC Adv. 7, 997–1007 (2017)
A. Khojastehnezhad, M. Rahimizadeh, H. Eshghi et al., Chin. J. Catal. 35, 376–382 (2014)
U. Kusampally, R. Pagadala, T. Damera et al., Chem. Biodivers. 20, e202300413 (2023)
M.E. Navgire, S.R. Bhitre, A.A. Yelwande et al., Russ. J. Org. Chem. 58, 394–404 (2022)
M. Sadeghi, A. Ezabadi, B. Omidi et al., Res. Chem. Intermed. 49, 1405–1425 (2023)
N. Hasannezhad, N. Shadjou, J. Mol. Recognit. 35, e2956 (2022)
F. Mohamadpour, Polycycl. Aromat. Compd. 41, 160–172 (2019)
F. Mohamadpour, Org. Prep. Proced. Int. 52, 64–68 (2020)
A. Yazdani-Elah-Abadia, M. Maghsoodloua, R. Mohebat et al., Chin. Chem. Lett. 28, 446–452 (2017)
I. Sehout, R. Boulcina, B. Boumoud et al., Synth. Commun. 47, 1185–1191 (2017)
F. Moeinpour, A. Khojastehnezhad, J. Chem. 9, 504–509 (2012)
M. Faisal, S. Shahid, S.A. Ghumro et al., Synth. Commun. 48, 462–472 (2018)
M.G. Dehbalaei, N. Foroughifar, H. Pasdaret et al., New J. Chem. 42, 327–335 (2017)
B. Sardar, R. Jamatia, D. Pal et al., Asian J. Org. Chem. 10, 2195–2204 (2021)
A. Davoodnia, A. Khojastehnezhad, N. Tavakoli-Hoseini, Bull. Korean Chem. Soc. 32, 2243 (2011)
A.K. Dutta, P. Gogoi, R. Borah, RSC Adv. 4, 41287–41291 (2014)
G. Lavanya, K. Venkatapathy, C.J. Magesh et al., Appl. Organomet. Chem. 33, 1–13 (2019)
S. Yü, S. Wu, X. Zhao et al., Res. Chem. Intermed. 43, 3121–3130 (2017)
X. Fan, Y. Li, X. Zhang et al., Heteroat. Chem. 18, 786–790 (2007)
N. Sahiba, A. Sethiya, J. Soni et al., J. Mol. Struct. 1268, 133676 (2022)
Z. Amiri, M. Malmir, T. Hosseinnejad et al., Mol. Catal. 524, 112319 (2022)
U.M. Mandle, L.A. Dhale, S.B. Godase et al., Ferroelectrics 598, 169–186 (2022)
L.N. Nasirmahale, F. Shirini, Y. Bayat et al., New J. Chem. 46, 23129–23138 (2022)
N. Biswas, D. Srimani, J. Org. Chem. 86, 9733–9743 (2021)
M.A. Zolfigol, N. Bahrami-Nejad, S. Baghery, J. Mol. Liq. 218, 558–564 (2016)
P. Choudhury, P. Ghosh, B. Basu, Mol. Divers. 24, 283–294 (2020)
J. Kour, M. Gupta, B. Chowhan et al., J. Iran. Chem. Soc. 16, 2587–2612 (2019)
S. Asgharnasl, R. Eivazzadeh-Keihan, F. Radinekiyan et al., Biol. Macromol. 144, 29–46 (2020)
G. Shinde, J. Thakur, Res. Chem. Intermed. 50, 817–838 (2023)
Acknowledgements
The authors gratefully acknowledge the financial support from the Department of Science Technology-Science and Engineering Research Board (DST-SERB) and the University Grants Commission (UGC) as a major research project. One of the authors Nilam Dhane thanks to Bharat Ratna Dr. C. N. Rao Research Laboratory Yashavantrao Chavan Institute of Science, Satara (Autonomous). A Lead College of Karmaveer Bhaurao Patil University, Satara for providing laboratory facilities. And also thankful to Rajarshi Chhatrapati Shahu College, Kolhapur.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
NSD: Conceptualization and collection of information and writing the manuscript, ACS: Collection of Information and Interpretation of the result, SRA: Collection of Information and Interpretation of the result, SM D: Helped to interpret the data, GKC: Helped to interpret the data, SPP: Helped to interpret the data, SBK: Supervision, Design and Implementation of the Research, KVG: Supervision, Design and Implementation of the Research.
Corresponding authors
Ethics declarations
Conflict of interest
The author' declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhane, N.S., Sapkal, A.C., Attar, S.R. et al. Synthesis of 1, 8-dioxodecahydroacridines via Hantzsch condensation using theophylline in an aqueous medium: an eco-friendly and bio-based approach. Res Chem Intermed 50, 1147–1160 (2024). https://doi.org/10.1007/s11164-023-05213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05213-1